| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Review
Volume 2, Number 1, June 2013, pages 1-9
Basic Therapeutic Principles and the Strategy in the Management of the External Anogenital Warts (Condylomas): A Review
Nader Husseinzadeha, b
aDepartment of Obstetrics and Gynecology, Division of Gynecologic Oncology University of Cincinnati, Cincinnati, Ohio. 45220, USA
bCorresponding address: 231 Albert Sabin Way, Cincinnati, OH 45267-0526, USA
Manuscript accepted for publication January 29, 2013
Short title: Therapeutic Principles and Strategy
doi: https://doi.org/10.4021/jcgo86e
- Abstract
- Introduction
- Prevalence
- Clinical Presentation
- Treatments
- Medical Treatments
- Investigational Treatments
- Surgical Treatment
- Cryotherapy
- Surgical Excision
- Laser Vaporization
- Discussion
- References
| Abstract | ▴Top |
Genital warts are a highly contagious sexually transmitted disease involving the anogenital area. They are considered to be benign proliferative conditions as a result of an infection with a low risk human papillomavirus (HPV). The goal of this paper is to review recent available clinical data, and present an overview and strategy in the diagnosis and treatment of genital warts. Med-line search was conducted and published papers related to anogenital warts were reviewed. Treatment results differ and are directly related to the characteristics of the lesion, the patient’s immune response and the expertise of the health provider. Smaller lesions are more amenable and respond to local treatment compared to larger and more extensive lesions. Although genital warts are generally a benign disease, however infection with certain types of HPV may increase the risk of premalignant disease. Treatment decisions are based on the number, location, morphology of the lesion and patients’ co-existent medical conditions and their preferences for available treatment modalities. Important issues in the diagnosis and management of genital warts are discussed and specific recommendations are presented.
Keywords: Genital warts; Condyloma; HPV; STD treatment
| Introduction | ▴Top |
External genital wart (Condyloma) is a sexually transmitted disease caused by human papillomavirus (HPV). The human papilloma viruses are small, nonenveloped and contain a double- strand DNA genome. More than 35 types of HPV infect the genital tract [1].
The HPV infects basal epithelium of the skin or mucous membrane by micro-abrasion. Then its DNA is introduced into a host cellular genome which results in abnormal cell proliferation and growth. Cell mediated immunity plays a major role in regression or progression of HPV infection. More than 90% of external genital warts are caused by HPV-types 6 and 11. Such viruses are considered to be non-oncogenic or low-risk viruses [2, 3]. Co- infection with high risk HPV types 16, 18, 31, 33, and 35 are occasionally found presenting as foci of high grade intraepithelial neoplasia in genital warts.
Human papillomavirus is a papovavirus. Its capside lacks an envelope which makes it resistant to various treatments. Typing of HPV according to genotype usually is determined by molecular hybridization technique [4], however, the use of HPV-DNA typing does not add any additional information, therefore, is not recommended for patients with external genital warts.
| Prevalence | ▴Top |
HPV infection is probably the most common sexually transmitted disease in the United State. Approximately 20 million Americans are currently infected with HPV. Another 6 million people become newly infected each year. At least 50% of sexually active men and women get HPV infection at some point [5]. According to the Center for Disease Control and Prevention (CDC), 18.3% of female adolescences in the US have HPV infection which represents a significant public health concern [6].
| Clinical Presentation | ▴Top |
Genital warts generally do not become clinically apparent until several months after inoculation with HPV (Latent). Genital warts have an indolent course and can be asymptomatic (subclinical). Depending on the size and location, they can be pruritic and painful (Clinical). The lesions usually are hyperkeratotic and appear as flat, papule-like, or spiked around the interoitus, perineum and anus. The lesion can be single, multiple or confluent like cauliflower .They can present simultaneously at multiple sites within the anogenital region (for example, cervix, vagina, urethra or anus) causing pain or burning sensation during urination (vulvar dysuria) and pain during intercourse (dysparunia).
It is very helpful to record the size, location, distribution, and morphology of the lesions. This will allow monitoring for the subsequent disappearance of the original lesions and/or to identify the appearance of any new lesions. All women with genital warts should have a pelvic exam with cervical-vaginal cytology, tests for sexually transmitted diseases (STD) and possible colposcopy for abnormal cytology to identify coexistence of cervical or vaginal neoplasia. Routine use of acetic acid for delineation of the lesion before biopsy is no longer recommended [7].
Patients with anogenital warts require a thorough examination of the oral cavity, urethral meatus, and anal mucosa.
Diagnosis of genital warts is made clinically by visual inspection. It may be helped by the application of acetic acid and colposcopy in subclinical cases.
Biopsy is indicated if the patient is immunocompromised or when the lesion is atypical (for example, pigmented, indurated, fixed, and ulcerated) or does not respond to therapy. Genital warts should be differentiated from condyloma Latum, Seborrheic keratosis, Lichen Planus, Verrucous carcinoma and micropapillomatosis labialis. Acanthosis with papillomatosis (elongation of rete pegs) of the epidermis, with hyperkeratosis and parakeratosis are the histologic hallmarks of genital warts, cytologically the cells have eccentric, pyknotic nucleus surrounded by a perinuclear halo which are known as koilocytes.
| Treatments | ▴Top |
There are a variety of treatment options for anogenital warts. No data shows the superiority of one treatment over another. The patient should be informed about the success rate and potential risk and complications of each treatment, particularly in immunocompromised patients. The treatment plan should also include referral of the patient’s sexual partners to a physician with an expertise or interest in diagnosis and treatment of genital warts since her partner has probably been infected even though there might not be gross visible lesions. Most therapeutic modalities require multiple treatments with frequent follow up .Treatment strategy should be based on the size, location, distribution and number of lesions. Small lesions located in moist or intertriginous area are more likely to respond to topical treatment. Pedunculated or exophytic lesions are easily removed with excision.
| Medical Treatments | ▴Top |
Tricholoric Acids (TCAs)
These agents destroy the lesion by chemical coagulation of the cellular protein. For treatment a small amount of solution should be applied by a health care provider only to the visible lesions and allow to dry well before the patient sits or stands up. Care should be taken in protecting the surrounding tissue by applying petroleum gel, lidocaine ointment, or zinc oxide cream. If some solution spills over, it should be immediately neutralized with liquid soap or sodium bicarbonate (baking soda powder). The treatment is repeated weekly if necessary. Small lesions may be treated with no skin protection and it must be allowed to dry. Large and keratinized lesions are commonly refractory to TCA and may require ablative therapies such as laser, electrosurgery or surgical excision [8].
Podophyllin derivatives
These topical drugs are antimitotic agents that arrest the formation of the mitotic spindles in metaphase, thus prevent cell division causing necrosis which destroys warts over time. Podophyllin derivatives should not be used on mucosal surface or in pregnancy and is contraindicated for patients who have hypersensitivity or intolerance to any component of the drug formulation.
Podophyllin resin
Podophyllin is a cytotoxic agent that inhibits tubulin polymerization [9].This solution is a compound with tincture of benzoin. For treatment; the health provider should apply the solution with a small Q-tip applicator on each individual warts. Podophyllin is extremely neurotoxic therefore care should be taken not to apply it on healthy tissue or on an open wound because of systemic absorption and neurotoxicity. Treatment can be repeated every 1 - 2 week as long as the lesions respond to treatment. For very extensive lesions involving larger areas, treating a portion of the lesions at a time is preferable and it may reduce the severity of side effects (for example. pain, inflammation, ulceration). Podophyllin is not a stable compound and has a wide range of reported effectiveness from 32% to 79% with a high rate of treatment failure [10].
Podophyllotoxin 0.5% gel or solution
Podophyllotoxin is a purified derivative of the podophyllin with a mechanism of action which includes antimitosis, inhibition of nucleoside transport, stimulation of macrophage proliferation, and the production of interlukin-1 and interlukin-2. The medication is applied twice a day for 3 days followed by 4 days of rest in between. For very extensive lesions involving larger areas, treating a portion of the lesions at a time is preferable not to exceed an area of 10 cm2. The treatment can be repeated for 4 cycles and the patient is advised to wash the area 6 - 8 hours after the treatment. Long term success rate in eradicating these lesions may vary from 40-90% with post treatment recurrence of 30% to 60% [11, 12].
Immunomodulators (Imiquimod creams)
These drugs stimulate cell-mediated immunity and induce production of cytokines with antiviral activity (namely Interferon- alpha and tumor necrosis factor) to destroy the HPV infected cells within the lesion. These drugs also activates immune cells via the toll-like receptor 7 (TLR 7)-MyD88 - dependent signaling pathway and presumably act through direct stimulation of dendritic and other immune cells [13, 14]. This immune modification mediates the indirect antiviral, antiproliferative and anti tumor activity of Imiquimod in vivo. In an attempt to improve the therapeutic efficacy, different concentrations of imiquimod were developed without increasing the side effects or reducing their efficacy. Imiquimode is a patient applied topical treatment which can cause redness, itching, burning, or bleeding of the treated area.
Imiquimod 2% cream
This drug is applied topically by the patient at bed time for 6 - 8 weeks. In a randomized, double-blind, placebo-controlled study to determine the clinical efficacy and tolerability of Imiquimod (2%) cream to treat genital warts, sixty women ranging between 18 and 45 years of age (mean 24.3) had twice daily application of imiquimod for 5 consecutive days. By the end of 6 weeks, the cure rate was 83.3% among treated patients while the placebo was only effective in one patient. Eight patients (13.3%) in the Imiquimod group experienced mild to moderate, non-objective, drug-induced symptoms with no dropouts. Among the 26 cured patients, five (19%) had a relapse after 11 months [15].
Imiquimod 3.75% cream
This drug is applied every night by the patient for as long as 8 weeks or until the lesion disappears. In two large phase III, double-blind, placebo-controlled trials [16], application of Imiquimod once a day for up to 8 weeks was associated with complete clearance of the warts in 28.3% of patients compared with 9.4% of the patients using the placebo cream. Among the patients who experienced complete clearance of warts, only 15% experienced a recurrence within 12 weeks. The most common adverse effects included erythema, erosion, excoriation, itching and burning. Less than 1% of patients discontinued the medication as a result of these side effects [17].
Imiquimod 5% cream
This drug is applied topically by the patient at bed time every other night for total of 3 applications per week and the treated area is washed 6 - 8 hours later. The regiment can be repeated weekly until the lesion clears but not longer than 16 weeks [18]. Comparing the efficacy of these two concentrations (3.75% versus 5%), the 3.75% formulation was found to be more effective, easy to use and well tolerated. In six trials with HIV-negative patients, complete clearance of warts at the end of treatment occurred in 51% of patients treated with Imiquimod cream and 6% in placebo treated patients. In four trials the reduction (at least 50%) of warts has occurred in 72% of Imiquimod treated patients and 20% of placebo treated patients. In three other trials complete clearance of warts at the end of treatment with no recurrence were 37% in Imiquimode treated and 4% in placebo group [19].
In a systematic review of Imiquimod for the treatment of external genital wart through Medline search (1966 - 2006) Jaffary, F. et al [20] reported that none of the trials compared imiquimod to other active therapy. Seven Trials compared Imiquimod (1, 2 or 5% cream) to placebo in immunocompetent patients of which only two trials used recommended dose and dosing regimen with a follow up of 12 weeks duration. The effectiveness of Imiquimod over other existing therapies for the treatment of external genital/perianal warts in these trials has not been established. In immunocompetent patients, there is sufficient evidence to conclude that imiquimod 5% cream applied three times a week for a maximum duration of 16 weeks compared to placebo provides a short term advantage in terms of complete clearance with no recurrence at the end of 12 weeks of follow-up. However there was a significantly increased adverse effect in patients treated with imiquimod 5%. They concluded that in immunocompromised patients (HIV positive) Imiquimod 5% cream provides an acceptable efficacy and safety on HIV patients. Cuisini et al [21] compared the efficacy of Imiquimod 5% in immunocompromised patients and those with normal immune function.
In HIV patients impaired cell-mediated immunity reduced dendritic cell activity and circulating interferon (IFN) inhibitors which may contribute to the decreased efficacy of Imiquimode. Therefore when patients with HIV treated for longer periods (up to 20 weeks) clearance rates increase and recurrence rates decrease [22, 23].
Sinecatechins ointment
Sinecatechin is a green tea extract which was found to be effective in treatment of genital warts; however, it has no known mechanism. Green tea catechins exert antiviral, antioxidative, antiproliferative and immune-stimulatory activity. It is supplied in 10% and 15% strength. It is applied 3 times a day to insure a thin layer covers the lesion. Treatment can be continued until the warts are cleared but no longer than 6 weeks. Medication should not be washed off and the individual should abstain from sexual intercourse. The most common side effects are: erythema, pruritis, burning, pain/discomfort, erosion, ulceration, edema and induration similar to other topical medications. If these reactions occur, treatment may need to be discontinued with the option of restarting after the reaction subsides. In a randomized study by Tatti et al [24], patients applied sinecatechins ointment (15% or 10%) or placebo three times daily for a maximum of 16 weeks or until all warts cleared. They were followed for a 12 weeks period of being treatment-free to assess recurrence. Complete clearance of all warts was observed in 57.2% of patients assigned to 15% sinecatechins ointment, 56.3% of patients assigned to 10% ointment and 33.7% of patients assigned to placebo (P < 0.001). During follow-up, recurrence of any wart was observed in 6.5%, 8.3%, and 8.8% in the sinecatechins ointment 15%, 10% and placebo groups respectively. In another randomized study Stockfletch et al [25], investigated the efficacy and safety of Polyphenon E (a proprietary extract of green tea leaves) 15% and 10% ointment on 503 immunocompetent patients. Complete clearance of all baseline and new anogenital warts was noted in 53% patients treated with Polyphenon E (PE) 15% ointment (P = 0.01), 51% for Polyphenon 10% (P = 0.01), and 37% of the patients for placebo (P = 0.03). Women responded better than men. Approximately 60% of women and 45% of men in both active groups achieved complete clearance of all warts. Time to complete clearance was comparable for both strengths of PE ointment. About 78% of all patients treated with either PE 15% or 10% ointment showed wart clearance rates of 50% or better. Less than 6% patients in the PE 15% and 4% of patients in the PE 10% ointment groups experienced wart recurrence during follow-up.
Interferons (INFs)
Interferons are cytokines that have anti-proliferative and pro-inflammatory properties with broad antiviral activities. They increase the immune response by activation of lymphocyte and macrophage activity. Intralesional and systemic Interferons have been evaluated with variable results. A study by Bonnez et al [26] compared two dosages of parentally administered interferon (IFN)-beta in combination with cryotherapy for the treatment of anogenital warts. Subjects were randomized to receive 2 × 10 or 4 × 10 IU/m2 subcutaneous INF-beta injections three times a week for a total of 6 weeks. Cryotherapy was administered concomitantly using aerosol liquid nitrogen at 10-day intervals. Systemic side- effects were more common in the high dose group than in the low dose group (P = 0.02). Using survival analysis, there was no significant difference between the two groups in rate of resolution of warts (P = 0.62). However, the rate of appearance of a new lesion during the study was significantly lower in the high dose group (P = 0.04).
Intralesional or systemic Interferon has been used either alone or in combination with other drugs namely podophyllin and oral isotretinoin [27, 28]. Interferons are expensive with related side effect when they are used systemically. They do not prove to be advantageous in terms of efficacy and therefore are not recommended for routine clinical use [29].
Chemotherapeutic agents
Fluorouracil 5% (5-Fu)
The 5- Fluorouracil (5-FU) is a cytotoxic and antiproliferative agent which interferes with DNA synthesis. A thin layer of 5-Fu cream is applied to cover the lesions twice daily for 2 - 4 weeks. The surrounding normal tissue should be protected with petroleum jelly (such as Vaseline) to prevent irritation. Little is known about recurrences when 5-Fu cream is used. In one study, recurrence rate was reported in 50% of patients who were followed beyond the initial study period [30]. In a randomized phase II study Swinehart et al [31] reported the result of their study on 359 patients with 1926 condylomata lesions. For all lesions treated with fluorouracil/epinephrine gel, the complete response (CR) rate was 77%. For all patients treated with fluorouracil/epinephrine gel, the CR rate was 61% (P < 0.002). They concluded that fluorouracil/epinephrine gel was a safe and effective treatment for condylomata acuminate. Fluorouracil is currently not recommended for treatment of genital warts because of its side effects and questionable therapeutic effectiveness.
Bleomycin
Bleomycin is an antibiotic which possesses antiviral and anti neoplastic properties. It inhibits DNA and protein synthesis. Bleomycin is not thought to bind directly to HPV but it causes acute tissue necrosis that may stimulate an immune response [32]. A double-blind, placebo-controlled study of warts recalcitrant twice to conventional treatments compared one to two intralesional injections of bleomycin to injections of normal saline. Plantar warts (60%), periungual warts (94%) and warts on extremities (95%) were cleared [33]. Five randomized, controlled trials have evaluated the effectiveness of bleomycin in treatment of warts; however, it is difficult to compare the results of these trials. Cure rates varied from 16% to 94%. Three trials demonstrated higher cure rates with bleomycin than with placebo, one showed no significant difference between bleomycin and placebo, and one showed higher cure rates with placebo than with bleomycin. Different concentrations of bleomycin also made no significant difference after 3 months [34, 35].
| Investigational Treatments | ▴Top |
Treatments that currently under investigation for general use are:
Cidofovir 1% gel
Cidofovir is an acyclic nucleoside phosphonate with an activity against DNA viruses. Snoeck et al [36] reported the result of their study on 30 patients. Nine of 19 (47%) who received Cidofovir 1% once daily dose had complete response compared to none in the placebo group. In a small study Matteeli et al [37], reported that Cidofovir resulted in 58% reduction in total wart area in patients with HIV compared to Placebo group (P = 0.02). Local mucosal reaction was common side effect. Topical Cidofovir has also been shown to be an effective adjuvant therapy to surgical treatment of genital warts. Coremans et al [38] reported that Cidofovir alone cured the lesions in 32% of the patients and induced partial regression in 60%. However, in smokers, complete resolution of the condylomata occurred only in 16.6 percent compared with 66 percent of nonsmokers (P = 0.03). Use of Cidofovir is limited because of high cost and compound preparation of the topical cream by pharmacists.
Therapeutic vaccines
The goal of vaccination is to protect against genital warts and reduce transmission and HPV related cancer. In a recent double-blind placebo-controlled study on patients with anogenital warts [39], three doses of HPV-6 L2E7 vaccine or placebo were administered along with either ablative therapy or Podophyllotoxin. Although a positive trend toward clearance was seen in patients infected with only HPV-6, the vaccine did not significantly increase the efficacy of conventional therapies, despite induction of adequate immune responses. Extensive HPV typing by polymerase chain reaction demonstrated that a majority of screened subjects (73.7%) were infected with HPV-6 and/or HPV-11 and that a large proportion (40.1%) were infected with multiple HPV types. Therefore, therapeutic vaccination failed to increase the efficacy of conventional therapies. Future therapeutic vaccine which includes DNA plasmids encoding COPV L1 E1or E2 protein that has already shown promise in animal models may be considered [40].
Intralesional immunotherapy
The principle of intralesional immunotherapy is to elicit topical immunity. When an antigen is injected into cutaneous warts, a cell-mediated immune response is activated, resulting in clearance of the warts. Intralesional injection of interferon-α has been tested in a randomized, double blind, multicenter trial in patients with genital warts that are recurrent or recalcitrant to other treatments. The interferon or placebo was injected twice weekly for up to 8 weeks. Complete clearance was seen in 62% of interferon-α treated as compared to 21% of placebo-treated patients [41]. In a pilot study using intralesional Mycobacterium vaccine for treatment of anogenital warts complete clearance rate was 88.9% [42]. Intralesional injection of mumps or candida may be a treatment option for recalcitrant warts that have not resolved with other therapies. One trial compared the intralesional injection of wart with candida or mumps antigen to cryotherapy of all warts .Complete wart clearance was achieved in 74% of immune individuals treated with mumps or candida antigen immunotherapy compared to 55% treated with cryotherapy [43, 44]. This approach may become first line therapy for patients with multiple or large warts or as a second line therapy for those who have failed traditional treatment.
Retinoids
Retinoids are vitamin A derivitives which have shown to disrupt epidermal cell growth and differentiation. They are potent immunomodulator and it has been shown that retinoids are anticarcinogenic, including apoptosis of initiated or transformed cells [45, 46].
Retinoids can be applied topically or systemically. Treatment of warts with tretionin (trans- retinoid acid) cream resulted in 85% clearance in a series of children as compared to 32% spontaneous clearance in control [47].
Retinoids have shown efficacy in the treatment of cervical intraepithelial neoplasia (CIN) .Two studies reported that retinoids were associated with regression of CIN2. One reported a greater complete regression of CIN2 over placebo, which was of borderline statistical significance, odds ratio (OR) = 0.5 (95% confidence interval (CI) 0.25-1.02%). The other study reported a non-significant dose-related trend towards increased rates of complete and partial regression compared with placebo. In all studies retinoids had no significant effect on regression of CIN3 [48].
Retinoids have not been investigated in the treatment of anogenital warts. However oral isotrenitonin has shown efficacy in treating recalcitrant condyloma of the cervix therefore it may represent a treatment option for patient with anogenital warts [49].
| Surgical Treatment | ▴Top |
Electrosurgy
Electrosurgical methods used the for treatment of genital warts are either elecrocautery, using direct current system which causes thermal coagulation, or loop electrosurgical excision (LEEP), using alternating current system that can excise and coagulate simultaneously. A local anesthesia is required for small lesions and general anesthesia for extensive lesions; both can be done in an office setting or as an outpatient surgery. A retrospective 5 years study of 213 patients with extensive anogenital warts treated by electrosurgery under anesthesia was undertaken to determine clearance and recurrence rates of the disease. One hundred and seventy six patients had single procedures, 35 underwent repeat procedures and two (1%) cleared spontaneously before surgery. Clearance of the warts was found by 3 months in 57% of the single procedure cases, 78% in repeat procedure patients and 61% in the whole sample (95% CI 52.4-68.8%). Recurrence rates were 24%, 23% and 24% (95%CI 16.9-31.2%) respectively [50].
| Cryotherapy | ▴Top |
Cryotherapy destroys the wart by freezing cellular water causing epidermal and dermal necrosis. A spray gun containing liquid nitrogen is used for anogenital warts and freezing is applied to each wart for about 10 - 20 seconds (two freezing/thaw method). Another method is using a cryoprobe which is a closed system by using nitrous oxide, carbon dioxide, or liquid nitrogen. Proper selection of the cryoprobe tip is important in order not to destroy normal surrounding tissue. Cryotherapy does not kill the virus, and one should exercise precautions against both the spread of virus from patient to patient by contaminated probes and liquid nitrogen reservoirs. Treatment can be repeated every 1 - 2 weeks. Clearance rates of up to 90% with a recurrence rate of 40% have been reported [51].
| Surgical Excision | ▴Top |
Surgical excision of external genital wart probably is the most appropriate and often effective treatment, it provides specimen for histological examination. It can effectively remove any size lesion in one session. Small lesions can be removed in the physician office under local anesthesia. Large lesions can be removed in an outpatient surgical setting under local or general anesthesia. Hemostasis can be accomplished with a topical sclerotic agent, light electrocoagulation of the wound base or approximation of the skin edges with suture. The clearance rate is not 100% and recurrence may occur at the margin of excision. Post surgical care should include local hygiene and pain control [52].
| Laser Vaporization | ▴Top |
Laser vaporization with CO2 is also another appropriate surgical method for treating both small and large genital warts lesions. A focused laser beam can be used to excise the wart, after which a defocused beam is used to vaporize the base of the wart. Depth of destruction is commonly limited to less than 1 mm and should not reach to dermal papilla which may result in hypo or hyperpigmentation of the skin and scaring. The cure rate has been reported 64-71% at 12 months. The hazards in using laser may be associated with the laser plume, since HPV-DNA has been demonstrated to be present in CO2 vapor. Because of the cost involved in most cases, laser ablation is not considered as a first line of treatment. The procedure is performed under local or general anesthesia depending on the size, number and location of lesions. The recurrence rate has been reported up to 50% [53, 54]. Schoenfeld et al [55] reported that 24 of 28 (86%) lesions treated with LEEP and 21 of 28 (75%) of lesions treated with laser showed no HPV DNA within 2 mm circumference of the treated lesions.
| Discussion | ▴Top |
Most sexually active women will be infected by HPV at some point of their lives and may not know about it. Within ongoing sexual relationship, both partners are usually infected and should be evaluated. Genital warts can have significant physical and psychosexual impact on the patient’s life style. The individual can develop feelings of guilt, anger, loss of self esteem and concern about future fertility and cancer. The role of the health care provider becomes very important to console the patient and insure appropriate diagnosis and treatment. Genital warts are commonly caused by low risk HPV viruses, however, patients with genital warts can also be exposed to high risk (oncogenic) HPV viruses. These individuals infected with high risk HPV should also be considered at risk for cervical, vaginal and vulvar neoplasia. Diagnosis is based on clinical examination and treatment can become very challenging. Recalcitrant warts that have been present for more than 6 months are more resistant to treatment than those less than 6 months. Clearance rate is higher among young and healthy individuals with short duration of infection. Remission can occur spontaneously however most of the time they will require therapy. Treatment may be surgical namely excision, cryotherapy and laser or medical with antimitotic agents, immune modulators or chemotherapeutic agents. As yet there is no single effective treatment for anogenital warts and there is no clear understanding how current treatments will affect transmission or recurrence of the disease. All sexually active women should be provided any or all medical information regarding HPV infection, transmission, treatment options, recurrence and prevention. Self-application of topical treatment can be recommended to patients with small lesions. Tri-choleric acid (TCA) should not be applied to large and multifocal lesions at one session. Few small lesions confined to one area or single large lesion can be removed surgically under local anesthesia. Those lesions that persist or re-occur soon after initial treatment require biopsy or surgical excision and change in treatment modality. In contrast new lesions appearing during treatment do not require change in treatment modality. Sometimes attempts are made to combine therapies in patients with recalcitrant and recurrent warts. However there is little evidence to support this type of treatment, therefore more information is needed on treatment efficacy and complications before making any recommendation.
A summary of recommended evaluation and treatment strategy is presented in Figure 1. It is important to inform patients about the long latent reappearance of genital warts. They should be encouraged to maintain their follow up visits with gynecologic exam and cervical-vaginal cytology but not more than women without warts. Prevention and protection against this sexually transmitted disease has been a major public health issue however there are other treatment modalities currently under investigation. Presently the most promising treatment against genital wart is type-specific HPV-vaccines.
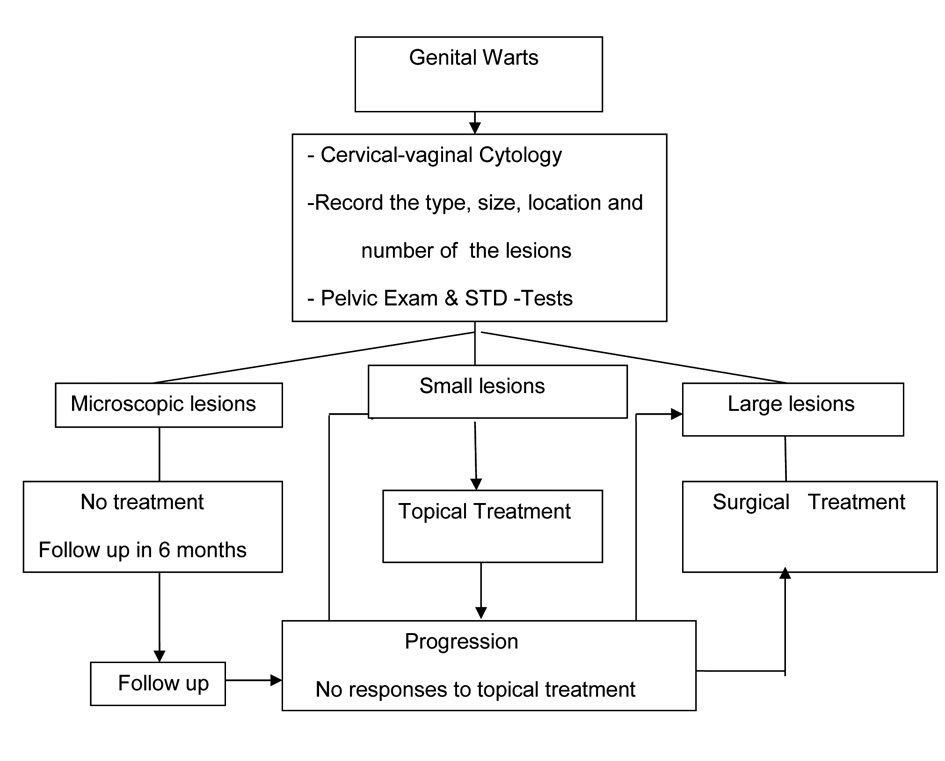 Click for large image | Figure 1. Recommended evaluation and management of patients with genital warts. |
Declaration
Author has no financial arrangement(s) to disclose.
| References | ▴Top |
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324(1):17-27.
doi pubmed - Doorbar J. The papillomavirus life cycle. J Clin Virol. 2005;32(Suppl 1):S7-15.
doi pubmed - Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
pubmed - de Villiers EM. Papillomavirus and HPV typing. Clin Dermatol. 1997;15(2):199-206.
doi - Center for Disease Control. Genital HPV infection- www.cdc.gov/std/HPV/STDfact-HPV.htm.
- Forhan SE, Gottlieb SL, Sternberg MR, Xu F, Datta SD, McQuillan GM, Berman SM, et al. Prevalence of sexually transmitted infections among female adolescents aged 14 to 19 in the United States. Pediatrics. 2009;124(6):1505-1512.
doi pubmed - Sexually transmitted disease treatment. www.cdc.gov/std/treatment/2010/genital.
- Beutner KR, Wiley DJ, Douglas JM, Tyring SK, Fife K, Trofatter K, Stone KM. Genital warts and their treatment. Clin Infect Dis. 1999;28(Suppl 1):S37-56.
doi pubmed - Saitoh T, Kuramochi K, Imai T, Takata K, Takehara M, Kobayashi S, Sakaguchi K, et al. Podophyllotoxin directly binds a hinge domain in E2 of HPV and inhibits an E2/E7 interaction in vitro. Bioorg Med Chem. 2008;16(10):5815-5825.
doi pubmed - Maw RD. Treatment of anogenital warts. Dermatol Clin. 1998;16(4):829-834, xv.
doi - Gunter J. Genital and perianal warts: new treatment opportunities for human papillomavirus infection. Am J Obstet Gynecol. 2003;189(3 Suppl):S3-11.
doi - Wiley DJ, Douglas J, Beutner K, Cox T, Fife K, Moscicki AB, Fukumoto L. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis. 2002;35(Suppl 2):S210-224.
doi pubmed - Tyring S, Conant M, Marini M, Van Der Meijden W, Washenik K. Imiquimod; an international update on therapeutic uses in dermatology. Int J Dermatol. 2002;41(11):810-816.
doi pubmed - Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196-200.
doi pubmed - Syed TA, Ahmadpour OA, Ahmad SA, Ahmad SH. Management of female genital warts with an analog of imiquimod 2% in cream: a randomized, double-blind, placebo-controlled study. J Dermatol. 1998;25(7):429-433.
pubmed - Wu J. Phase 3 Study of Imiquimod Creams in the Treatment of External Genital warts. Clinical trials.gov (Graceway Pharmaceuticals, LLC updated April 2011).
- Yan J, Chen SL, Wang HN, Wu TX. Meta-analysis of 5% imiquimod and 0.5% podophyllotoxin in the treatment of condylomata acuminata. Dermatology. 2006;213(3):218-223.
doi pubmed - Carrasco D, vander Straten M, Tyring SK. Treatment of anogenital warts with imiquimod 5% cream followed by surgical excision of residual lesions. J Am Acad Dermatol. 2002;47(4 Suppl):S212-216.
doi pubmed - Moore RA, Edwards JE, Hopwood J, Hicks D. Imiquimod for the treatment of genital warts: a quantitative systematic review. BMC Infect Dis. 2001;1:3.
doi pubmed - Jaffary F, Musini V, Niluforoushrzadeh MA, Bassett K. Systemic review of imiquimod for the treatment of external genital wart. Int J Pharmacology 2007; 3:1-10.
doi - Cusini M, Salmaso F, Zerboni R, Carminati G, Vernaci C, Franchi C, Locatelli A, et al. 5% Imiquimod cream for external anogenital warts in HIV-infected patients under HAART therapy. Int J STD AIDS. 2004;15(1):17-20.
doi pubmed - Herrera S, Correa LA, Wolff JC, Gaviria A, Tyring SK, Sanclemente G. Effect of imiquimod in anogenital warts from HIV-positive men. J Clin Virol. 2007;39(3):210-214.
doi pubmed - Arrese J, Paquet P, Claessens N, Pierard-Franchimont C, Pierard G. Dermal dendritic cells in anogenital warty lesions unresponsive to an immune-response modifier. J Cutan Pathol. 2001;28(3):131-134.
doi pubmed - Tatti S, Swinehart JM, Thielert C, Tawfik H, Mescheder A, Beutner KR. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts: a randomized controlled trial. Obstet Gynecol. 2008;111(6):1371-1379.
doi pubmed - Stockfleth E, Beti H, Orasan R, Grigorian F, Mescheder A, Tawfik H, Thielert C. Topical Polyphenon E in the treatment of external genital and perianal warts: a randomized controlled trial. Br J Dermatol. 2008;158(6):1329-1338.
doi pubmed - Bonnez W, Oakes D, Bailey-Farchione A, Choi A, Hallahan D, Corey L, Barnum G, et al. A randomized, double-blind trial of parenteral low dose versus high dose interferon-beta in combination with cryotherapy for treatment of condyloma acuminatum. Antiviral Res. 1997;35(1):41-52.
doi - Armstrong DK, Maw RD, Dinsmore WW, Morrison GD, Pattman RS, Watson PG, Nathan PM, et al. A randomised, double-blind, parallel group study to compare subcutaneous interferon alpha-2a plus podophyllin with placebo plus podophyllin in the treatment of primary condylomata acuminata. Genitourin Med. 1994;70(6):389-393.
pubmed - Cardamakis EK, Kotoulas IG, Dimopoulos DP, Stathopoulos EN, Michopoulos JT, Tzingounis VA. Comparative study of systemic interferon alfa-2a with oral isotretinoin and oral isotretinoin alone in the treatment of recurrent condylomata accuminata. Arch Gynecol Obstet. 1996;258(1):35-41.
doi pubmed - Wiley DJ, Beutner KR. Genital Warts . Clin Evidence 2000;3:764-774.
- Krebs HB. Treatment of extensive vulvar condylomata acuminata with topical 5-fluorouracil. South Med J. 1990;83(7):761-764.
doi pubmed - Swinehart JM, Sperling M, Phillips S, Kraus S, Gordon S, McCarty JM, Webster GF, et al. Intralesional fluorouracil/epinephrine injectable gel for treatment of condylomata acuminata. A phase 3 clinical study. Arch Dermatol. 1997;133(1):67-73.
doi pubmed - Girao L, Franca I, Macedo H, Ornelas C, Nunes M, Araujo C, Mansinho K. Treatment of oral condylomata acuminata in a HIV-1 patient with bleomycin. J Eur Acad Dermatol Venereol. 2000;14(4):321-322.
doi pubmed - Shumer SM, O'Keefe EJ. Bleomycin in the treatment of recalcitrant warts. J Am Acad Dermatol. 1983;9(1):91-96.
doi - Gibbs S, Harvey I, Sterling JC, Stark R. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2003;(3):CD001781.
- Bigby M, Gibbs S. Warts. Clin Evid. 2005;(14):2091-2103.
- Snoeck R, Bossens M, Parent D, Delaere B, Degreef H, Van Ranst M, Noel JC, et al. Phase II double-blind, placebo-controlled study of the safety and efficacy of cidofovir topical gel for the treatment of patients with human papillomavirus infection. Clin Infect Dis. 2001;33(5):597-602.
doi pubmed - Matteelli A, Beltrame A, Graifemberghi S, Forleo MA, Gulletta M, Ciravolo G, Tedoldi S, et al. Efficacy and tolerability of topical 1% cidofovir cream for the treatment of external anogenital warts in HIV-infected persons. Sex Transm Dis. 2001;28(6):343-346.
doi pubmed - Coremans G, Margaritis V, Snoeck R, Wyndaele J, De Clercq E, Geboes K. Topical cidofovir (HPMPC) is an effective adjuvant to surgical treatment of anogenital condylomata acuminata. Dis Colon Rectum. 2003;46(8):1103-1108; discussion 1108-1109.
doi pubmed - Vandepapeliere P, Barrasso R, Meijer CJ, Walboomers JM, Wettendorff M, Stanberry LR, Lacey CJ. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis. 2005;192(12):2099-2107.
doi pubmed - Moore RA, Walcott S, White KL, Anderson DM, Jain S, Lloyd A, Topley P, et al. Therapeutic immunisation with COPV early genes by epithelial DNA delivery. Virology. 2003;314(2):630-635.
doi - Friedman-Kien AE, Eron LJ, Conant M, Growdon W, Badiak H, Bradstreet PW, Fedorczyk D, et al. Natural interferon alfa for treatment of condylomata acuminata. JAMA. 1988;259(4):533-538.
doi pubmed - Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: an open label pilot study. J Eur Acad Dermatol Venereol. 2008;22(9):1089-1093.
doi pubmed - Johnson SM, Roberson PK, Horn TD. Intralesional injection of mumps or Candida skin test antigens: a novel immunotherapy for warts. Arch Dermatol. 2001;137(4):451-455.
pubmed - Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with mumps, Candida, and Trichophyton skin test antigens: a single-blinded, randomized, and controlled trial. Arch Dermatol. 2005;141(5):589-594.
doi pubmed - Jason J, Archibald LK, Nwanyanwu OC, Sowell AL, Buchanan I, Larned J, Bell M, et al. Vitamin A levels and immunity in humans. Clin Diagn Lab Immunol. 2002;9(3):616-621.
pubmed - Niles RM. Recent advances in the use of vitamin A (retinoids) in the prevention and treatment of cancer. Nutrition. 2000;16(11-12):1084-1089.
doi - Kubeyinje EP. Evaluation of the efficacy and safety of 0.05% tretinin cream in the treatment of plane warts in Arab children. J Dermatol Treat 1996;7:21-22.
doi - Helm CW, Lorenz DJ, Meyer NJ, Rising WR, Wulff JL. Retinoids for preventing the progression of cervical intra-epithelial neoplasia. Cochrane Database Syst Rev. 2007;(4):CD003296.
- Georgala S, Katoulis AC, Georgala C, Bozi E, Mortakis A. Oral isotretinoin in the treatment of recalcitrant condylomata acuminata of the cervix: a randomised placebo controlled trial. Sex Transm Infect. 2004;80(3):216-218.
doi pubmed - Challenor R, Alexander I. A five-year audit of the treatment of extensive anogenital warts by day case electrosurgery under general anaesthesia. Int J STD AIDS. 2002;13(11):786-789.
doi pubmed - Tabrizi SN, Garland SM. Is cryotherapy treating or infecting? Med J Aust. 1996;164(5):263.
pubmed - Sterling JC, Handfield-Jones S, Hudson PM. Guidelines for the management of cutaneous warts. Br J Dermatol. 2001;144(1):4-11.
doi pubmed - Gloster HM, Jr., Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32(3):436-441.
doi - Savoca S, Nardo LG, Rosano TF, D'Agosta S, Nardo F. CO(2) laser vaporization as primary therapy for human papillomavirus lesions. A prospective observational study. Acta Obstet Gynecol Scand. 2001;80(12):1121-1124.
doi pubmed - Schoenfeld A, Ziv E, Levavi H, Samra Z, Ovadia J. Laser versus loop electrosurgical excision in vulvar condyloma for eradication of subclinical reservoir demonstrated by assay for 2'5' oligosynthetase human papillomavirus. Gynecol Obstet Invest. 1995;40(1):46-51.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.







