| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 3, Number 1, February 2014, pages 22-29
Improved Early Prediction of Preterm Pre-Eclampsia by Combining Second Trimester Maternal Serum Alpha-Fetoprotein and Uterine Artery Doppler
Rebecca Allena, c, Shemoon Marleena, Luxmilar Velauthara, Kevin Harringtonb, Joseph Aquilinaa
aFetal Medicine Centre, Barts Health NHS Trust, South Tower, Royal London Hospital, Whitechapel, London, E1 1BB, UK
bThe Portland Hospital, 205-209 Great Portland St, London, W1W 5AH, UK
cCorresponding author: Rebecca Allen, Fetal Medicine Centre, 8th Floor, South Tower, Royal London Hospital, Whitechapel, London, E1 1BB, UK
Manuscript accepted for publication January 17, 2014
Short title: AFP and UAD to Predict PE
doi: https://doi.org/10.14740/jcgo223w
| Abstract | ▴Top |
Background: Pre-eclampsia (PE) is a leading cause of maternal and perinatal morbidity and mortality. One of the primary aims of antenatal care is to identify women at high risk and provide them with prophylactic treatment and more intensive surveillance. Current identification is based mainly on maternal characteristics, which is not specific and sensitive enough to be an ideal screening test highlighting the need for an alternative. We evaluated the combination of second trimester maternal serum alpha-fetoprotein (MSAFP) and uterine artery Doppler (UAD) studies for the prediction of PE.
Methods: A total of 724 women had MSAFP and UAD measured. The presence of notches and Resistance Index were measured. ROC’s were created for MSAFP and UAD alone and in combination. Sensitivities for the two outcomes were compared for a fixed specificity of 94% for PE and 97% for preterm PE.
Results: A total of 41 women (5.7%) developed PE. The sensitivity of using UAD (bilateral notches/mean RI ≥ 0.735) was 60.9% and for MSAFP was 24.4% (≥ 2.0 MoM). The combination of UAD (bilateral notches/mean RI ≥ 0.55) and MSAFP (≥ 1.2 MoM), didn’t improve the sensitivity of UAD for PE for the same specificity; 17 women (2.4%) developed preterm PE. The sensitivity using UAD (bilateral notches/mean RI ≥ 0.75) was 29.4% and for MSAFP (≥ 2.6 MoM) was 5.9%. The combination of UAD (bilateral notches/mean RI ≥ 0.55) and MSAFP (≥ 1.6 MoM), improved the sensitivity for preterm preeclampsia to 64.7% (OR 52.17 (CI 17.81 - 152.84)). The improvement in sensitivity for the combined method was statistically significant compared to MSAFP (P < 0.01) or UAD (P < 0.02) alone.
Keywords: Alpha-fetoprotein; Uterine artery Dopplers; Pre-eclampsia; Screening; Second trimester
| Introduction | ▴Top |
Pre-eclampsia (PE) remains a leading cause of maternal and perinatal morbidity and mortality. The ultimate aim of early identification of a high-risk group of women is to reduce these risks. One of the pathophysiological mechanisms associated with the development of PE is partial or complete failure of trophoblastic invasion [1]. Doppler ultrasound provides indirect evidence of this and is used as a screening test for PE and related complications. Current evidence supports measuring uterine artery Dopplers (UAD) at around 20 - 24 weeks where the detection rate for pregnancies that subsequently develop PE requiring early delivery is 50-70% for a false positive rate of 5% [2].
Alpha-fetoprotein is a glycoprotein produced by the fetal liver and gastrointestinal tract. Up to the third month the majority of fetal production arises from the yolk sac. Maternal serum alpha-fetoprotein (MSAFP) is influenced by a combination of fetal production, clearance through fetal kidney and any perturbation of the placental interface between the fetus and the mother.
The association between elevated second trimester MSAFP in chromosomally normal fetuses and subsequent development of PE was first reported by Gordon et al [3]. Walters et al reported that 13% of women with elevated MSAFP developed PE compared to 1% of the women with normal MSAFP [4]. Williams et al compared 201 women with unexplained elevated MSAFP (≥ 2.0 MoM) with 211 women with normal MSAFP [5]. A significant association was found between elevated MSAFP and PE, adjusted risk ratio (ARR) being 3.8. Several other studies have also confirmed these findings [6-8].
Brazerol reported that the explanation for the association between elevated MSAFP and adverse pregnancy outcome is not clear, but is probably a marker of placental dysfunction, including partial placental abruption, feto-maternal bleeding and abnormal implantation [9]. Both UAD and MSAFP are indirect markers of impaired placentation, which forms the basis of the pathophysiology of PE. The hypothesis for our study is that the combination of second trimester MSAFP and UAD screening at 20 weeks will improve the screening efficacy of either investigation for the subsequent development of PE and other predefined pregnancy complications.
| Methods | ▴Top |
This was a prospective study where seven hundred and twenty four consecutive unselected women who had MSAFP measured as part of the serum-screening programme for trisomy 21 over a twelve-month period were included. Exclusion criteria were: multiple pregnancies, diabetic pregnancies, hypertension diagnosed before 20 weeks gestation, pregnancies with a prenatal/post-natal diagnosis of a chromosomal or structural abnormality. These women formed part of a larger group of women who were offered UAD studies, as part of a prospective study, when they presented for a routine anomaly scan between the nineteenth and twenty-first week of pregnancy [10]. Ethics approval was obtained from the local ethics committee. The investigators were blinded to the serum biochemistry results unless they were identified as screen positive for trisomy 21 (Risk ≥ 1 in 300). The department caters for a high risk inner-city multi-racial community.
All the serum samples for measurement of MSAFP levels were collected between 15 and 19 weeks gestation. MSAFP was assayed using the Technicon Immuno 1R system (Bayer, Switzerland), which adopts the heterogeneous sandwich magnetic separation assay. There is a significant inverse relationship between maternal weight and MSAFP levels between 15 - 20 weeks, the levels were therefore corrected for weight and expressed as Multiples of the Median (MoM), as previously described for other analytes such as hCG and oestriol [11, 12]. The method for obtaining the uterine artery flow velocity waveforms is described in our previous paper [10]. Women with bilateral notches/mean RI ≥ 0.55 or unilateral notches/mean RI ≥ 0.65 were offered four weekly growth scans up till 36 weeks due to the increased risk of fetal growth restriction [13, 14]. If a reduction in the growth velocity was suspected, reduced liquor or the umbilical artery PI was above the 90th centile, scans were booked every one to two weeks. The results of the growth scans were available to clinicians but the results of the UAD were not routinely reported unless specifically requested.
Demographic data collected prospectively is shown in Table 1. The main outcome measure was the development of PE. The development of pregnancy induced hypertension was based on the definitions given by Davey and McGilliviray: pregnancy induced hypertension was defined as the occurrence in a previously normotensive and non-proteinuric women of [1] a diastolic blood pressure greater than or equal to 90 mmHg on at least two consecutive occasions at least 4 hours apart after the 20th week of gestation or [2] a diastolic blood pressure greater than or equal to 110 mmHg on a single occasion after the 20th week of gestation [15]. PE was diagnosed when pregnancy induced hypertension was associated with significant proteinuria which was defined as > 300 mg proteinuria on 24 hour urinary collection or the appearance of at least ++ on protein stick-testing on two separate occasions, four hours apart, in the absence of a urinary tract infection. Women who required delivery before 37 completed weeks of gestation as a direct consequence of PE on the mother or fetus, were analyzed as a subset and subsequently referred to as ‘preterm PE’. The secondary outcome was the development of any of the following complications: pregnancy induced hypertension, PE, abruption, pre-term delivery, stillborn baby, a baby who died in the neonatal period, a baby born small for gestational age (< 5th centile). Outcome data was collected from the patient notes and labor ward. During the data collection the investigators were blinded to the MSAFP and Doppler data. The sensitivity, specificity, positive predictive value, negative predictive value, odds ratios and positive likelihood ratios for the subsequent development of PE, preterm PE and the above pregnancy complications were calculated for the following methods: 1), method 1: MSAFP cut-offs between 0.50 MoM and 4.0 MoM; 2), method 2: Bilateral notches with variable RI cut-offs (between the 50th and 90th centile) and all unilateral notches with mean RI ≥ 0.65 (fixed variable); 3), method 3: Bilateral notches with mean RI ≥ 0.55 (50th centile) and all unilateral notches/ mean RI ≥ 0.65 (fixed variable) combined with MSAFP cut-offs between 0.50 MoM and 4.0 MoM.
 Click to view | Table 1. Demographic Characteristics of Study Population |
Receiver operator characteristic (ROC) curves were created for each method. Arbitrary cut-offs were created, to ensure smooth ROC curves and to enable us to compare the sensitivity of the different methods for fixed specificities. The data was analyzed using a Windows based SPSS statistical package version 8.1.3. Statistical significance was assessed using Chi-squared test or Fisher’s exact test where cell counts were small. The Chi-squared and Mann-Whitney U tests were used to compare categorical and continuous demographic data in women with known outcome and those lost to follow-up. McNemar’s test was used to assess the statistical significance of the differences in sensitivities obtained for fixed specificities. The positive Likelihood Ratios (LR) was calculated for the main outcomes. The positive likelihood ratio for an abnormal result is the ratio of women who have an abnormal test result among those who have the defined disease to women who have an abnormal test but do not have the disease; this equals sensitivity/(1-specificity). Statistical significance was set at P < 0.05.
| Results | ▴Top |
The study recruitment period ran over two years from November 1997 to November 1999. MSAFP assays of 773 women were available for analysis. UAD studies were performed at a mean gestation of 20.5 weeks (range 18 - 22) and uterine artery waveforms were obtained from both sides in all women studied; 4 pregnancies were excluded: two because of subsequent development of hydrops; one because of Down’s syndrome and one was born with ambiguous genitalia; 45 pregnancies were lost to follow up leaving 724 (93.6%) outcomes available for analysis; 41 (5.7%) women developed PE, of which 17 (2.4%) required delivery before 37 weeks gestation as a direct consequence of PE (Fig. 1). There were no significant differences in race, booking weight or blood pressure between the women with a known outcome and those lost to follow-up. Other demographic characteristics are listed in Table 1.
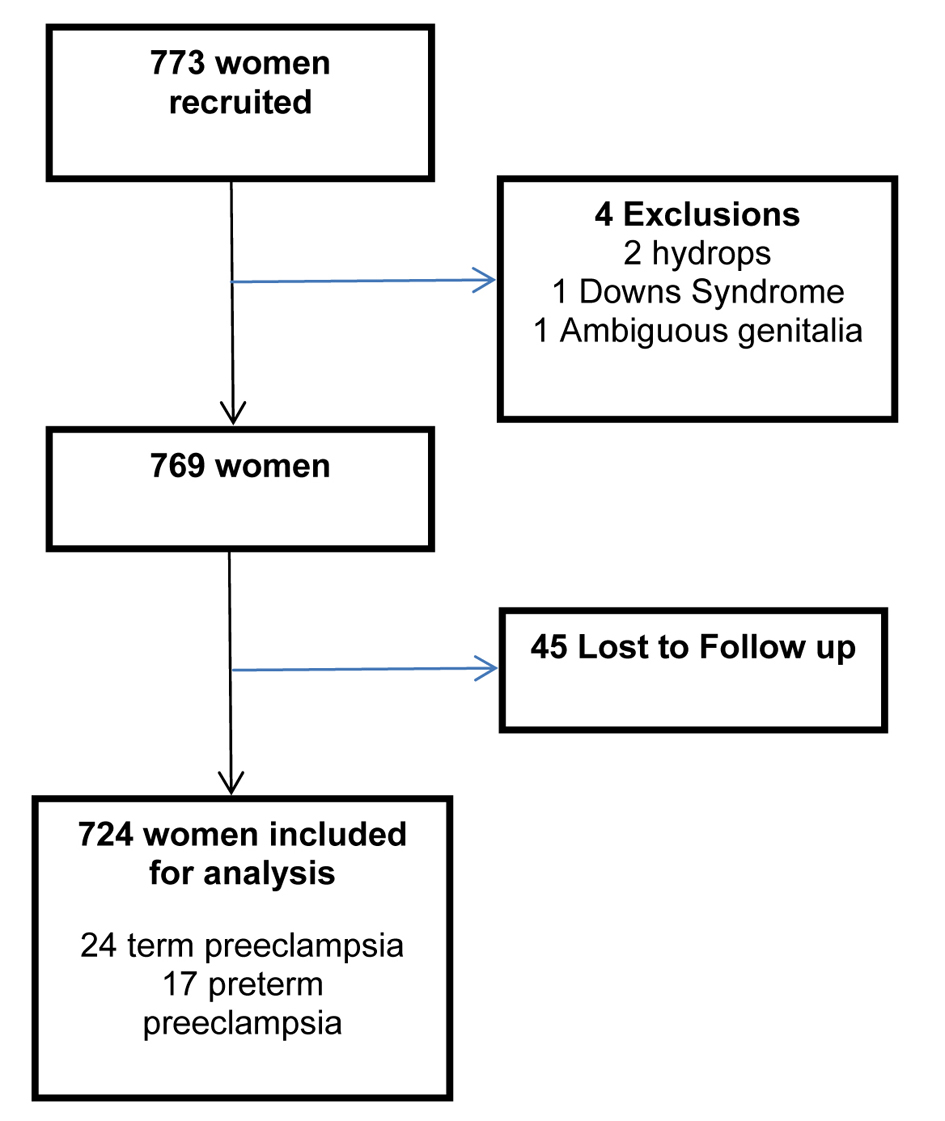 Click for large image | Figure 1. Flow chart demonstrating included women. |
For the outcome of PE; in method 1 the sensitivity, specificity, positive predictive value, negative predictive value and odds ratio for MSAFP as a stand-alone screening test at various cut-offs between ≥ 0.50 MoM and ≥ 4.0 MoM are presented as a ROC (Fig. 2). The highest sensitivity and specificity were obtained using MSAFP ≥ 1.6 MoM: sensitivity 41.5%, specificity 87.8%, positive predictive value 17.0%, negative predictive value 96.2% and odds ratio of 5.12 (95% confidence intervals (CI) 2.64 - 9.93).
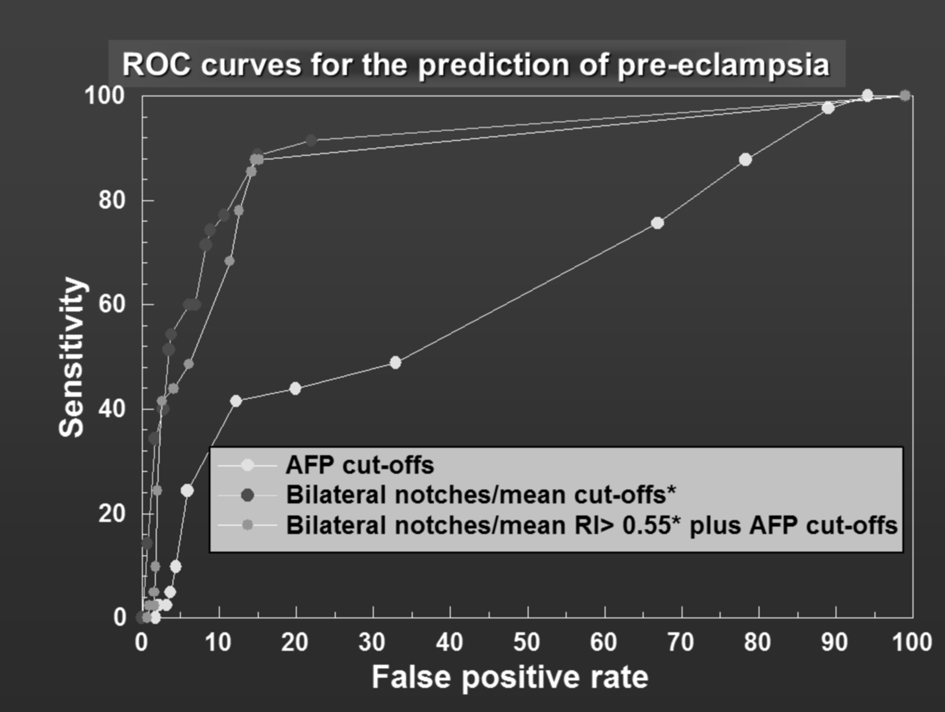 Click for large image | Figure 2. ROC curves for the prediction of pre-eclampsia. |
The same methodology was applied to UAD (method 2) and the results are presented as a ROC curve (Fig. 2). Compared to MSAFP, UAD performed significantly better as a stand-alone test across all specificities (Fig. 2). For a specificity of 94.7% (false-positive rate closest to 6) the sensitivity of UAD (bilateral and unilateral notches/mean RI ≥ 0.67) was significantly better than MSAFP (≥ 2.0 MoM) (60.9% vs. 24.4%) P < 0.001. When method 3 (combined method) was compared to method 2 (UAD alone), the sensitivity was not better. For a specificity of approximately 94% (93.9%), the sensitivity of UAD combined with MSAFP (≥ 1.2 MoM) was 48.8%, this is less than the sensitivity of UAD alone, but better than MSAFP alone (24.4%, P < 0.001). The likelihood ratio was 4.1 in method 1, 8.6 in method 2 and 8 in method 3. The results are summarized in Table 2.
 Click to view | Table 2. Prediction of Pre-Eclampsia and Preterm Pre-Eclampsia Using Uterine Artery Doppler and MSAFP Alone and in Combination |
The same methodological analysis was applied for preterm PE. For similar specificities, the sensitivity of MSAFP as a stand-alone test for preterm PE was consistently higher than that for all pre-eclamptics including preterm ones (for example, for a specificity of 97%, sensitivity 5.9% for preterm PE vs 2.4% for PE). The results for the prediction of preterm PE using the three methods are presented as a ROC curve (Fig. 3). UAD as a stand-alone test (method 2) again performed significantly better than MSAFP (method 1) across all specificities (Fig. 3). The sensitivity for method 3 (combined method) was generally better for specificities ≥ 96.6% compared to method 2. For a specificity of approximately 97%, the sensitivity of the combined method (bilateral notches/mean RI ≥ 0.55 and MSAFP ≥ 1.6 MoM) was significantly better than UAD (bilateral notches/mean RI ≥ 0.75) alone or MSAFP ≥1.6 alone (64.7% vs. 29.4% and 5.9%). The positive likelihood ratio goes from 1.90 for MSAFP to 9.8 for uterine Doppler and to 19.01 for the combined method. The results are tabulated in Table 2.
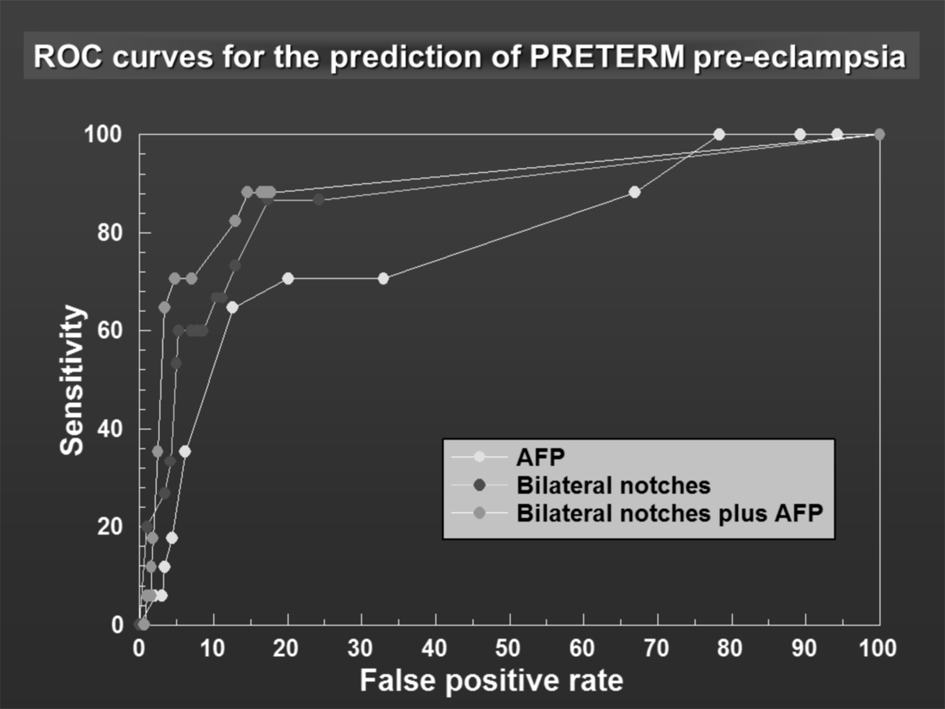 Click for large image | Figure 3. ROC curves for the prediction of PRETERM pre-eclampsia. |
The receiver operator characteristic curves (Fig. 2, 3) demonstrate the effect of combining both tests by a shift of the peak of the curve to the left-hand side.
| Discussion | ▴Top |
This study reports a statistically significant improvement in the screening efficacy for preterm PE when UAD studies at 20 weeks are combined with MSAFP data obtained between 15 - 19 weeks gestation. Using a cut off of AFP ≥ 1.6 MoM the PPV was 48.6% for all preeclampsia and 31.4% for preterm preeclampsia.
Strengths and limitations
A summary of similar studies to ours are shown in Table 3. All but one of these studies selected women based on them having elevated MSAFP levels or abnormal UADs, these studies also had a much smaller sample size [16-18]. The advantage of our study is that we had a larger unselected population of 724 women whom all had MSAFP and UADs measured. The study performed by Audibert et al is most similar to ours. They demonstrated a PPV of 21% for all PE when uterine artery notching was combined with an AFP > 1.5MoM [7]. Our study shows a higher PPV. This is may be due to the higher prevalence of PE in our population (5.7% vs 1.95%). On the basis of published evidence, if trials are to determine the real potential of therapies such as aspirin or calcium, a screening test in the first half of pregnancy with a high sensitivity, positive predictive value (PPV) (over 40%) and positive likelihood ratio (over 12) appears to be essential. Our study’s results are comparable to these (PPV 48.6%) with earlier identification at 20 weeks.
 Click to view | Table 3. Summary of Related Studies |
Our study identified nearly two out of three pregnancies that developed PE requiring preterm delivery, with a PPV of 31.4% for a false positive rate of approximately 3%. A lower prevalence (average of 3%), will result in lower PPVs, assuming that similar sensitivities and specificities are obtained. The positive likelihood ratio of 19.02 would, however, still support the use of this method of screening in populations with low prevalence.
The combination of MSAFP and UAD did not improve the sensitivity of the test for all cases of PE. This may indicate different etiological mechanisms between PE and preterm PE. Recent studies on placental pathology have suggested that early onset disease is more likely to be associated with abnormal villous and vascular morphology, whereas in late onset disease these are not dissimilar to controls. There is evidence that late onset disease compared with early onset is more likely to be related to impaired glucose metabolism and a hyperdynamic low peripheral resistance (as opposed to a low cardiac output vasoconstricted) maternal cardiovascular profile [19]. If late PE is not associated with impaired placentation then it is therefore not surprising that AFP doesn’t perform as well as a marker for all preeclampsia when compared to preterm preeclampsia. Melchiore showed a difference in first trimester UAD indices between preterm and term preeclampsia also supporting a different pathophysiology [20]. In their study the majority of pregnancies complicated by preterm preeclampsia showed Doppler evidence of reduced uteroplacental perfusion, predisposing to placental hypoxaemia, oxidative stress and trophoblastic apoptosis. It is thought that in pregnancies complicated by preeclampsia at term there are late atherosclerotic changes in the spiral arterioles but they are thought to have undergone normal transformation by the trophoblast in the first trimester [20]. Another argument could be that women presenting with preeclampsia at a later gestational age may have less severe trophoblastic pathology than those presenting preterm, with less severe trophoblastic/placentation changes, indirect measures such as AFP and UADs may not detect these mild changes.
A limitation of our study is that in women whom had bilateral notches/mean RI ≥ 0.55 and those with unilateral notches/mean RI ≥ 0.65 serial growth scans up till 36 weeks were offered. More intensive monitoring in this group may lead to earlier delivery due to the detection of IUGR which may have developed before the clinical features of PE leading to an underestimation in the screening performance of our test. However, it may have also increased the detection of disease that may have otherwise been missed, leading to an increase in preterm deliveries for PE/IUGR.
Our study excluded women with diabetes and chronic hypertension. Previous meta-analyses examining the use of aspirin for the prevention of preeclampsia, have shown a significant reduction when given to high risk women at an early gestation. It could be argued that we shouldn’t have excluded these women and it may be interesting in future work to compare the effectiveness of UADs and AFP as a screening tool for preeclampsia in a high risk population versus a low risk population. However, the focus of our study was to identify women that would otherwise be considered as low risk as high risk women would be commenced on aspirin prophylaxis regardless of any biochemical or biophysical tests. The National Collaborating centre for Women’s and Children’s Health has issued guidelines on routine prenatal care recommending that at the first visit a woman’s level of risk for PE should be evaluated by a series of maternal characteristics, for example, age, weight to identify those at high risk and allow intensive monitoring. However this approach would falsely classify two thirds of women as being high risk and in need of intensive monitoring [21]. Poon et al examined the effectiveness of combining maternal characteristics and previous history into an algorithm derived by multivariate analysis to estimate the individual patient specific risk of preeclampsia and found one third of pregnancies developing preeclampsia were detected [22]. These studies highlight the need for a more sensitive and specific screening test for detecting women at risk of developing preeclampsia.
Clinical implications
The ultimate aim of identifying a high-risk group is the institution of effective prophylactic therapies that can prevent or palliate the underlying pathophysiology in PE. When aspirin was given to women selected by UAD at 24 - 26 weeks (positive predictive value 41%), the number of pre-eclamptics requiring early delivery was significantly less compared to women on placebo, but there was no overall reduction in the incidence of preeclampsia [23]. Bujold et al performed a meta-analysis of 34 RCTs comparing the reduction in preeclampsia if aspirin was started prior to, or after, 16 weeks in women at risk of preeclampsia. They found a significant decrease in the incidence of preeclampsia when low dose aspirin therapy was commenced prior to 16 weeks (RR 0.47, 95% CI 0.34 - 0.65) but no significant decrease when it was commenced after 16 weeks (RR 0.81, 95% CI 0.63 - 1.03) [24]. Roberge also performed a meta-analysis examining RCTs where aspirin was initiated at or before 16 weeks to a high-risk population and showed an 89% reduction in pre-eclamptics delivered before 37 weeks gestation but had no effect on the risk of term preeclampsia [25]. Although our study has shown second trimester MSAFP and UADs have a reasonable PPV for detecting preeclampsia (PPV 48.6% in our study), these meta-analyses’ highlight the need to start prophylaxis as early as possible. If the screening efficacy of MSAFP and UADs can be replicated in the first trimester aspirin can be given earlier when it is likely to further improve outcomes. In future work it would be interesting to examine the preventative effect of aspirin when given to a group of women that would have otherwise be considered low risk but were considered high risk based UADs and AFP measurements.
Our findings, though promising, are limited by the fact that the emphasis of PE screening has now shifted to the first trimester, therefore, MSAFP and UADs need to be validated in larger prospective studies in the first trimester. There is good evidence to suggest from Akolekar’s recent paper that multi-marker streaming in combination with first trimester Doppler constitutes a very effective early screening test [21]. The drawbacks of such a strategy are the high economic costs of testing for many markers. It is estimated that this test would cost around £30/patient excluding machine maintenance, staffing costs etc. AFP screening at the time of first trimester screening is cheap (about £1) and may also help with improvement of T21 screening. [26, 27]. The combination of MSAFP with first trimester UAD needs to be validated in larger prospective studies of unselected populations.
Acknowledgments
We would like to thank Professor Nicholas Wald and Mr Wayne Huttley (Wolfson Institute of Preventative Medicine, Charterhouse Square, London) for providing us with the serum MSAFP data corrected for gestation and maternal weight.
Funding
None.
Disclosures
None.
| References | ▴Top |
- Meekins JW Pijenborg R, Hanssens M., McFayden IR, Van Asshe A. A study of placental bed spiral arteries and trophoblastic invasion in normal and severe pre-eclamptic pregnancies. BJOG. 1994;101.
- Papageorghiou AT, Yu CK, Cicero S, Bower S, Nicolaides KH. Second-trimester uterine artery Doppler screening in unselected populations: a review. J Matern Fetal Neonatal Med. 2002;12(2):78-88.
doi pubmed - Gordon YB, Grudzinskas JG, Kitau MJ, Usherwood MM, Letchworth AT, Chard T. Fetal wastage as a result of an alpha-fetoprotein screening programme. Lancet. 1978;1(8066):677-678.
doi - Walters BN, Lao T, Smith V, De Swiet M. alpha-Fetoprotein elevation and proteinuric pre-eclampsia. Br J Obstet Gynaecol. 1985;92(4):341-344.
doi pubmed - Williams MA, Hickok DE, Zingheim RW, Luthy DA, Kimelman J, Nyberg DA, Mahony BS. Elevated maternal serum alpha-fetoprotein levels and midtrimester placental abnormalities in relation to subsequent adverse pregnancy outcomes. Am J Obstet Gynecol. 1992;167(4 Pt 1):1032-1037.
doi - Dugoff L, Hobbins JC, Malone FD, Vidaver J, Sullivan L, Canick JA, Lambert-Messerlian GM, et al. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005;106(2):260-267.
doi pubmed - Audibert F, Benchimol Y, Benattar C, Champagne C, Frydman R. Prediction of preeclampsia or intrauterine growth restriction by second trimester serum screening and uterine Doppler velocimetry. Fetal Diagn Ther. 2005;20(1):48-53.
doi pubmed - Dehghani-Firouzabadi R, Tayebi N, Ghasemi N, Tahmasbi Z. The association between second-trimester maternal serum alpha-fetoprotein in 14-22 weeks and adverse pregnancy outcome. Acta Med Iran. 2010;48(4):234-238.
pubmed - Brazerol WF, Grover S, Donnenfeld AE. Unexplained elevated maternal serum alpha-fetoprotein levels and perinatal outcome in an urban clinic population. Am J Obstet Gynecol. 1994;171(4):1030-1035.
doi - Aquilina J, Thompson O, Thilaganathan B, Harrington K. Improved early prediction of pre-eclampsia by combining second-trimester maternal serum inhibin-A and uterine artery Doppler. Ultrasound Obstet Gynecol. 2001;17(6):477-484.
doi pubmed - Wilkins-Haug L. Unexplained elevated maternal serum alpha-fetoprotein: what is the appropriate follow-up? Curr Opin Obstet Gynecol. 1998;10(6):469-474.
doi pubmed - Wald NJ, Cuckle HS, Densem JW, Nanchahal K, Royston P, Chard T, Haddow JE, et al. Maternal serum screening for Down's syndrome in early pregnancy. BMJ. 1988;297(6653):883-887.
doi pubmed - Kurdi W, Campbell S, Aquilina J, England P, Harrington K. The role of color Doppler imaging of the uterine arteries at 20 weeks' gestation in stratifying antenatal care. Ultrasound Obstet Gynecol. 1998;12(5):339-345.
doi pubmed - Harrington K, Copper D, Lees C, Hecher K, Campbell S. Uterine Doppler ultrasound: the importance of bilateral notches in the prediction of uteroplacental complications. Ultrasound Obstetrics and Gynaecology. 1996;7.
pubmed - Davey DA, MacGillivray I. The classification and definition of the hypertensive disorders of pregnancy. Am J Obstet Gynecol. 1988;158(4):892-898.
doi - Jauniaux E, Gulbis B, Tunkel S, Ramsay B, Campbell S, Meuris S. Maternal serum testing for alpha-fetoprotein and human chorionic gonadotropin in high-risk pregnancies. Prenat Diagn. 1996;16(12):1129-1135.
doi - Chung JE, Cho JS, Han SS, Park YW, Kim JW. Uterine artery Doppler velocimetry in the prediction of adverse obstetric outcomes in unexplained MSAFP elevations. Yonsei Med J. 2000;41(1):17-21.
pubmed - Konchak PS, Bernstein IM, Capeless EL. Uterine artery Doppler velocimetry in the detection of adverse obstetric outcomes in women with unexplained elevated maternal serum alpha-fetoprotein levels. Am J Obstet Gynecol. 1995;173(4):1115-1119.
doi - Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009;53(5):812-818.
doi pubmed - Melchiorre K, Wormald B, Leslie K, Bhide A, Thilaganathan B. First-trimester uterine artery Doppler indices in term and preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32(2):133-137.
doi pubmed - Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11-13 weeks. Prenat Diagn. 2011;31(1):66-74.
doi pubmed - Poon LC, Kametas NA, Chelemen T, Leal A, Nicolaides KH. Maternal risk factors for hypertensive disorders in pregnancy: a multivariate approach. J Hum Hypertens. 2010;24(2):104-110.
doi pubmed - Bower SJ, Harrington KF, Schuchter K, McGirr C, Campbell S. Prediction of pre-eclampsia by abnormal uterine Doppler ultrasound and modification by aspirin. Br J Obstet Gynaecol. 1996;103(7):625-629.
doi pubmed - Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, Forest JC, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 Pt 1):402-414.
doi pubmed - Roberge S, Villa P, Nicolaides K, Giguere Y, Vainio M, Bakthi A, Ebrashy A, et al. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther. 2012;31(3):141-146.
doi pubmed - Haddow JE, Palomaki GE, Knight GJ, Williams J, Miller WA, Johnson A. Screening of maternal serum for fetal Down's syndrome in the first trimester. N Engl J Med. 1998;338(14):955-961.
doi pubmed - Bredaki FE, Wright D, Matos P, Syngelaki A, Nicolaides KH. First-trimester screening for trisomy 21 using alpha-fetoprotein. Fetal Diagn Ther. 2011;30(3):215-218.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.







