| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 4, Number 2, June 2015, pages 217-225
A Clinical Experience to Assess Safety and Efficacy of Fixed Dose Combination of Clindamycin and Clotrimazole in the Treatment of Patients With Mixed Bacterial and Fungal Vaginosis
Manish Maladkara, b, Shrikant Patila, Suvarcha Sooda
aAristo Pharmaceuticals Pvt. Ltd, 23-A, Shah Industrial Estate, Off Veera Desai Road, Andheri (W), Mumbai 400053, India
bCorresponding Author: Manish Maladkar, Aristo Pharmaceuticals Pvt. Ltd, 23-A, Shah Industrial Estate, Off Veera Desai Road, Andheri (W), Mumbai 400053, India
Manuscript accepted for publication May 21, 2015
Short title: Clindamycin and Clotrimazole for Vaginosis
doi: http://dx.doi.org/10.14740/jcgo331w
| Abstract | ▴Top |
Background: The aim of the study was to evaluate the safety and efficacy of clindamycin (100 mg) + clotrimazole (200 mg) suppository in the treatment of mixed bacterial and fungal vaginosis.
Method: Five hundred patients with clinical diagnosis of infective vaginosis entered this multi-center, prospective, open-label, non-comparative study. A fixed dose combination of clindamycin (100 mg) and clotrimazole (200 mg) suppository was inserted intravaginally for three nights.
Results: Symptoms of infective vaginosis decreased significantly within 3 days of treatment initiation. On day 3 of treatment, 84.8% patients had normal and odorless vaginal discharge, and the mean vaginal burning and irritation score declined by 92.4% and 94.8% respectively. Also, there was remission of vulvar, cervical and vaginal erythema in more than 85% of patients. The physicians and patients rated treatment efficacy as good to very good improvement and tolerability to the treatment was regarded as good to excellent. No serious adverse events were reported.
Conclusion: The present study demonstrates that the fixed dose combination of clindamycin and clotrimazole is effective and well tolerated in the treatment of mixed vaginosis due to bacterial and fungal origin.
Keywords: Vaginosis; Fungal; Bacterial; Clindamycin; Clotrimazole; Fixed dose combination; Suppository
| Introduction | ▴Top |
Infections of the vagina and vulva are among the most common medical problems seen in general practice. The three most common causes of infective vaginosis are bacterial vaginosis (BV), candidiasis and trichomoniasis. Vaginosis due to simultaneous infection with at least two pathogens (mixed infection, e.g. BV in patient with vulvovaginal candidiasis (VVC)) is also highly prevalent and makes up to 30% of all cases [1].
BV is a common condition affecting millions of women annually, and is associated with numerous health problems including pre-term labor resulting in low birth weight, pelvic inflammatory disease and acquisition of HIV. Malodorous vaginal discharge may be the only symptom of BV; however, many affected women are asymptomatic [2]. Among pregnant women, BV has an incidence of 15-20% [3]. During BV, beneficial lactic acid-producing bacteria (lactobacilli) are replaced by amine-producing anerobic bacteria. Although BV is considered a polymicrobial condition, one of the predominant bacterial species is Gardnerella vaginalis [4].
Among the many causes of vaginitis, VVC is the second most common after BV and is diagnosed in up to 40% of women with vaginal complaints in the primary care setting [5]. An estimated 75% of women will have at least one episode of VVC, and 40-45% will have two or more [6]. Risk factors for VVC include pregnancy (30-40%), use of high estrogen content contraceptives, antibiotics, steroids and chemotherapeutics. Numerous studies have shown Candida albicans is the causative organism in 85-90% symptomatic episodes of vaginal candidosis [7].
Diagnosis of vaginitis is made using a combination of symptoms, physical examination, pH of vaginal fluid, microscopy, and the whiff test [8]. Certain cases where the cause of vaginosis is not confirmed and may be of mixed origin, a single form of medication capable of treating bacterial, fungal and mixed infections may be beneficial.
Systemic or local antibiotic therapy remains the standard regimen for the treatment of BV [9]. Antimicrobial compounds like clindamycin are effective against anerobic bacteria and also help in relieving symptom [10]. In the treatment of infections of fungal origin like VVC, the current available option is antifungal agents. Many preparations are effective in the treatment of candidiasis. A vaginal imidazole, inserted nightly is recommended as the standard treatment for VVC [11]. Clotrimazole is a frequently used imidazole in the treatment of VVC with a cure rate of 80-90%.
The objective of this study was to evaluate the safety and efficacy of fixed dose combination of clindamycin and clotrimazole in the treatment of patients with mixed bacterial and fungal vaginosis.
| Materials and Methods | ▴Top |
Design of investigation
A prospective, open-label, non-comparative and multicentric post-marketing surveillance study including 500 patients with clinical diagnosis of infectious vaginosis was conducted at five different centers across India by qualified investigators. Safety assessment was on basis of occurrence and severity of any adverse event. At baseline, demographic data, medical history, previous treatment and physical examination data were collected from all patients. Patients were evaluated at baseline and then at second and third day for treatment efficacy and evaluation were based on improvement of parameters like consistency and odor of vaginal discharge, burning, and irritation, vulvar, vaginal and cervical erythema. At the end of the treatment, global assessment of efficacy and tolerability was confirmed by both physicians as well as patients.
Study medication
A fixed dose combination of clindamycin (100 mg) + clotrimazole (200 mg) suppository was inserted intravaginally for three consecutive nights.
Patient’s selection
Symptomatic females 18 years of age or older with clinical diagnosis of infective vaginosis were included in the study. Subjects agreed not to douche or use any intra-vaginal products during the study period (including tampons, medications and devices). However, certain pregnant females or those planning to be pregnant, history of hypersensitivity to clotrimazole or clindamycin, menstruating females or those expected to be menstruating during days of the study period and females using antifungal or antibiotic therapy within 14 days prior to enrolment were excluded from the study.
Clinical assessment parameters
The efficacy of combination was evaluated at second and third day after treatment initiation. Vaginal discharge of patients with vaginosis was evaluated based on its consistency (where white, thin and flocculent was normal discharge; whereas white curdy discharge as well as thin grayish white homogeneous discharge coating the vaginal wall indicating vaginosis), odor (fishy/offensive odor = 1 or odorless = 2). Presence and absence of vaginal burning and irritation were tested and graded as mild, moderate and severe. Occurrence of other symptoms like vulvar, vaginal and cervical erythema was confirmed. Safety was evaluated on the basis of any adverse event reported by the patient or observed by the investigator. Adverse events were graded as mild, moderate and severe based on severity and onset. The global assessment of efficacy and tolerability of treatment was done by the physician and patient at the end of the study. Efficacy was evaluated based on scale: 1 = very good improvement, 2 = good improvement, 3 = moderate improvement and 4 = negligible improvement and tolerability assessment was based on scale: 1 = excellent, 2 = good, 3 = fair and 4 = poor.
Statistics
The data were pooled and results were analyzed using parametric and non-parametric tests which were two-tailed and P < 0.05 was considered significant.
| Results | ▴Top |
Demographical profile
Five hundred patients were enrolled in study, of whom 14 patients dropped. Therefore, a total of 486 patients were evaluated. Overall demographic break-up is depicted in Table 1. Age of the patients evaluated was in range of 21 - 48 years and the weight in the range of 41 - 77.5 kg. Physical examination of parameters like temperature, pulse rate, respiratory rate and blood pressure was within the normal limits at baseline as depicted in Table 2.
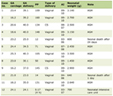 Click to view | Table 1. Demographical Data |
 Click to view | Table 2. Physical Examination Parameters |
Evaluation of efficacy
Evaluation of vaginal discharge consistency
At baseline 331 patients had thin gray/white homogeneous discharge coating the vaginal wall and 155 patients had a white curdy discharge. None of the subjects had white, thin flocculent, i.e. normal vaginal discharge. On second day after treatment initiation, 60.3% women had normal vaginal discharge, whereas on third day of treatment 87.4% patients had normal vaginal discharge. Thus statistically significant numbers of patients were benefited post- treatment with P < 0.05 (Fig. 1).
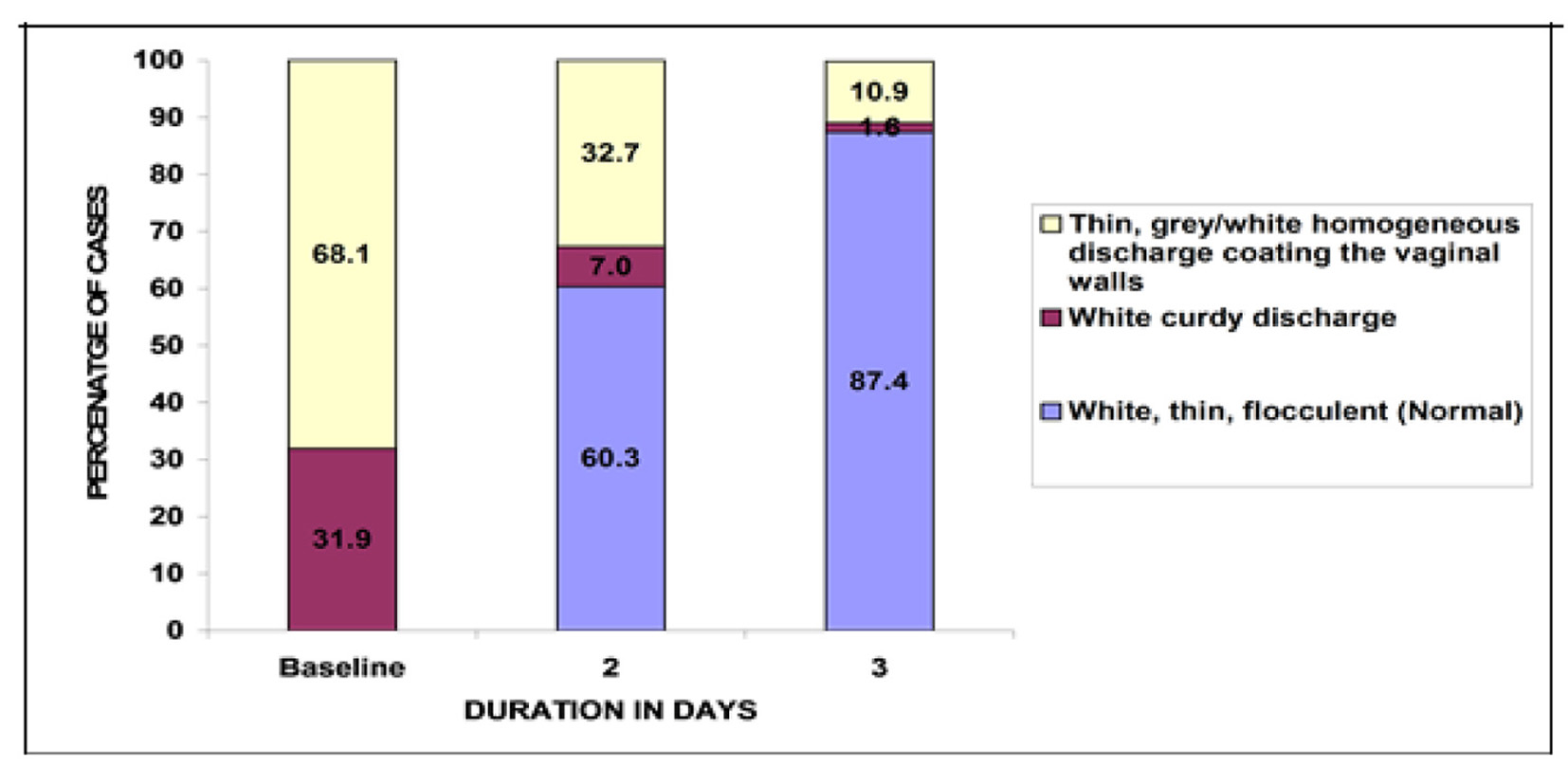 Click for large image | Figure 1. Changes in the number of patients with vaginal discharge consistency after the treatment. |
Evaluation of vaginal odor
At baseline, all patients had vaginal discharge with a fishy/offensive odor. Post-treatment a statistically significant number of patients (P < 0.05) reported odorless vaginal discharge. Of patients, 68.9% at second day and 84.8% at third day reported odorless vaginal discharge (Fig. 2).
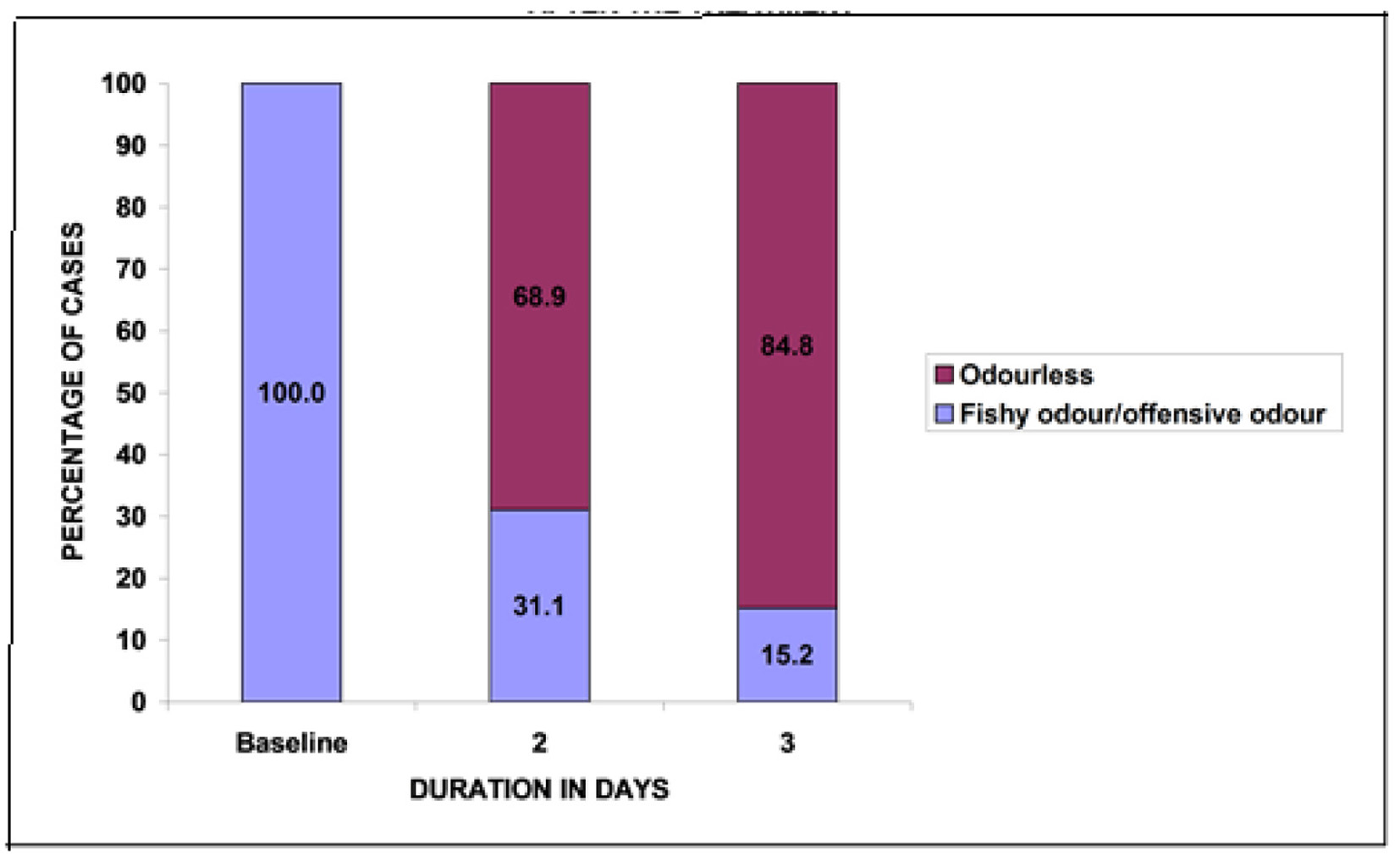 Click for large image | Figure 2. Changes in the number of patients with vaginal discharge odor after treatment. |
Evaluation of vaginal burning score
The mean score of vaginal burning was 1.84 at baseline. At second and third visit the mean score of vaginal burning showed a significant fall of 50.5% at second visit and 92.4% at third visit from baseline, P < 0.05 (Fig. 3).
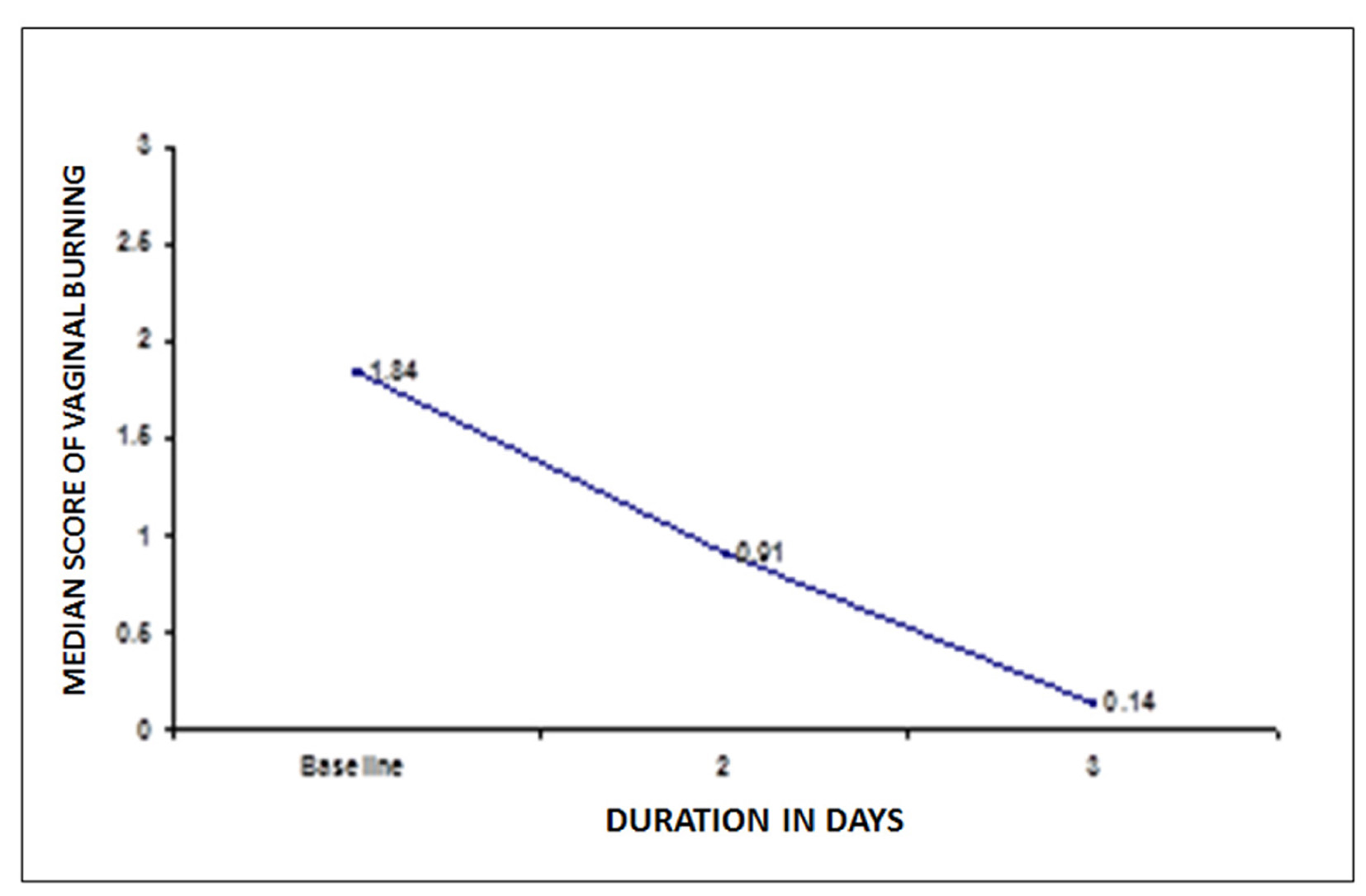 Click for large image | Figure 3. Changes in mean score of vaginal burning after treatment. |
Evaluation of vaginal irritation score
Mean vaginal irritation score was 1.74 at baseline. At second and third visit the vaginal irritation score declined to 50.6% and 94.8% respectively post-treatment, P < 0.05 (Fig. 4).
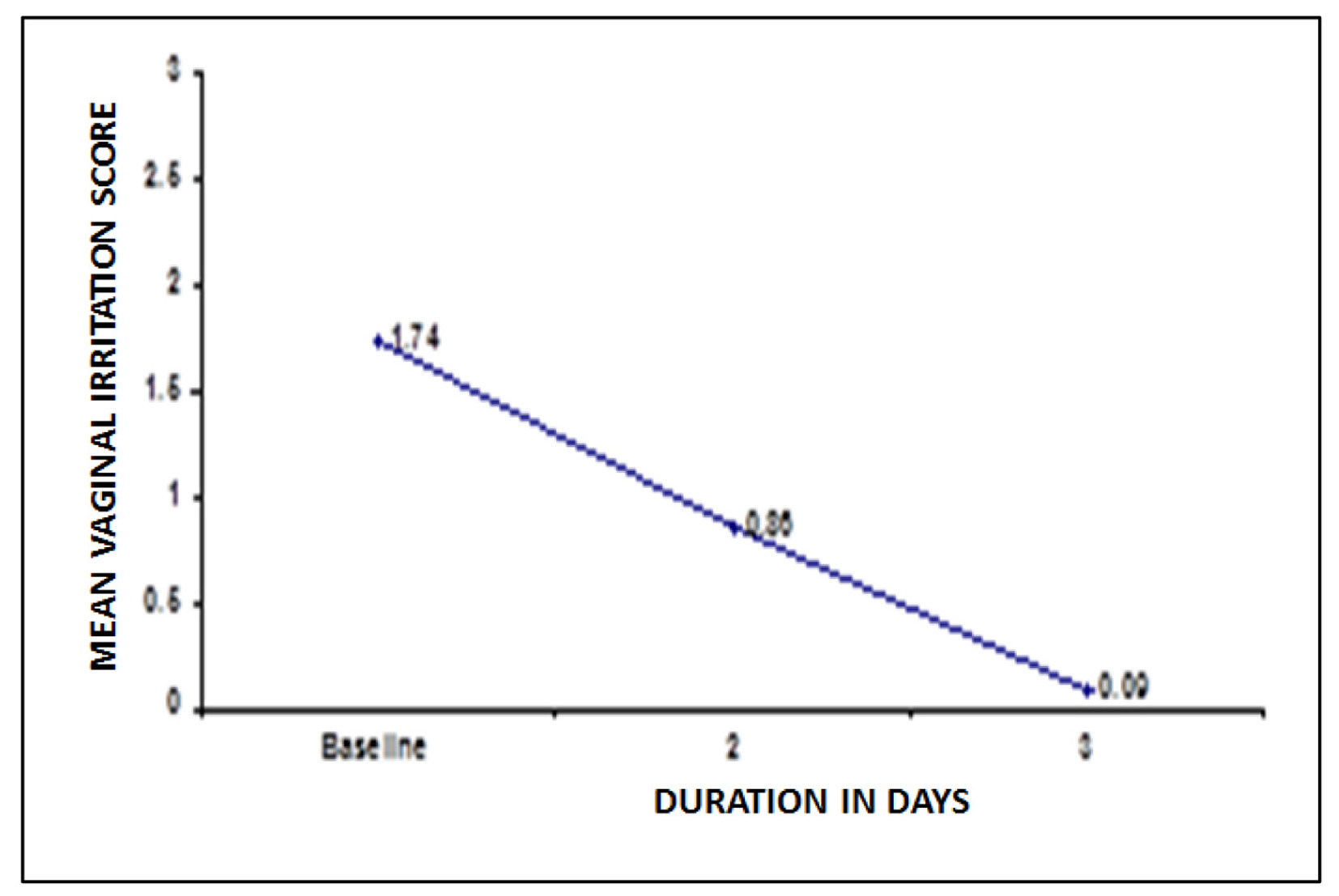 Click for large image | Figure 4. Changes in vaginal irritation score after treatment. |
Evaluation of vulvar erythema
At baseline, a total of 429 patients reported vulvar erythema. Post-treatment, on second and third visit, 72% and 87.7% females reported absence of vulvar erythema. Thus, a statistically significant increase was reported in number of females with no vulvar erythema post-treatment, P < 0.05 (Fig. 5).
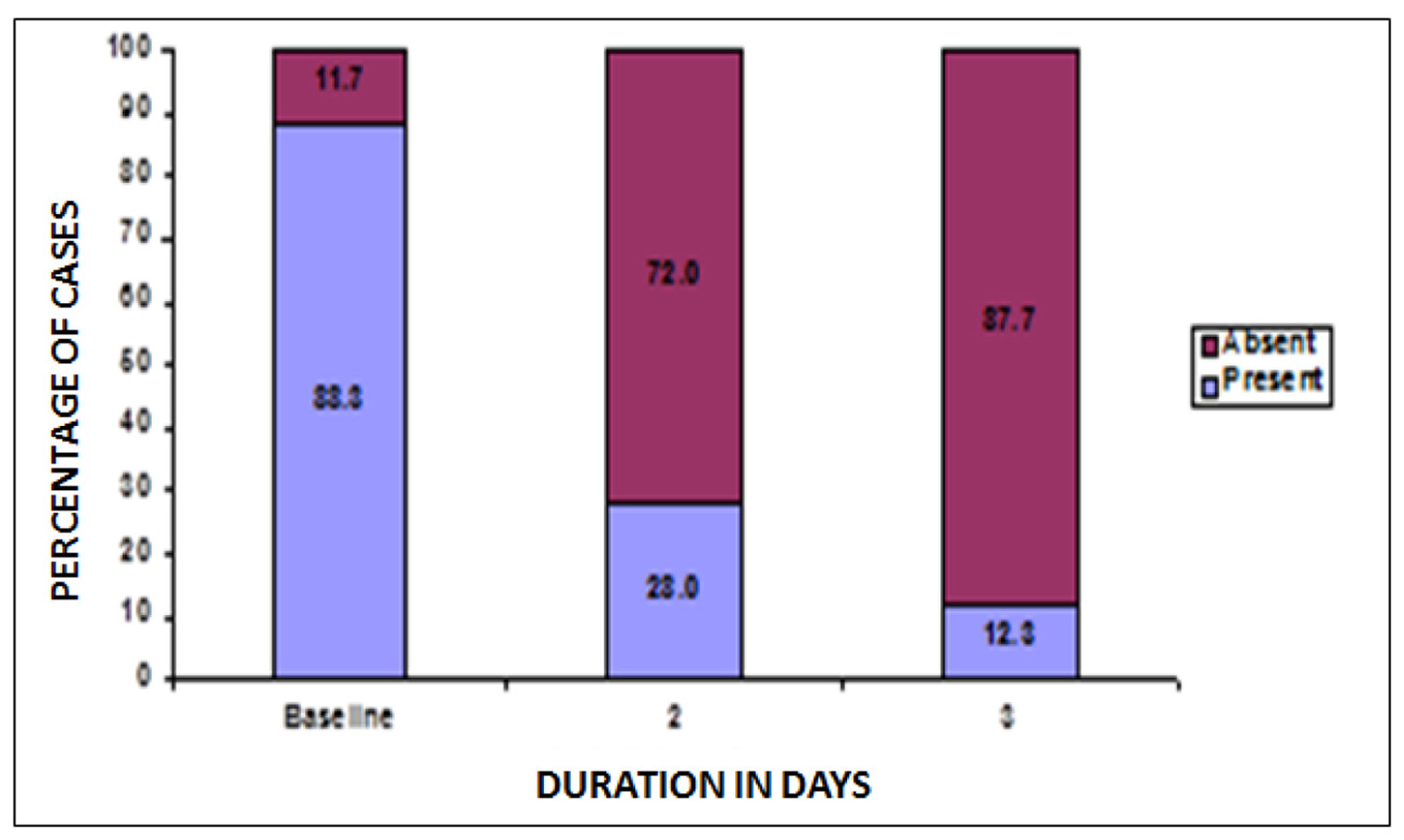 Click for large image | Figure 5. Changes in patients with vulvar erythema after treatment. |
Evaluation of vaginal erythema
At baseline, a total of 434 patients reported vaginal erythema. Post-treatment, on second and third visit 77.2% and 90.1% females reported absence of vaginal erythema. Thus, a statistically significant increase was reported in number of females with no vulvar erythema post-treatment, P < 0.05 (Fig. 6).
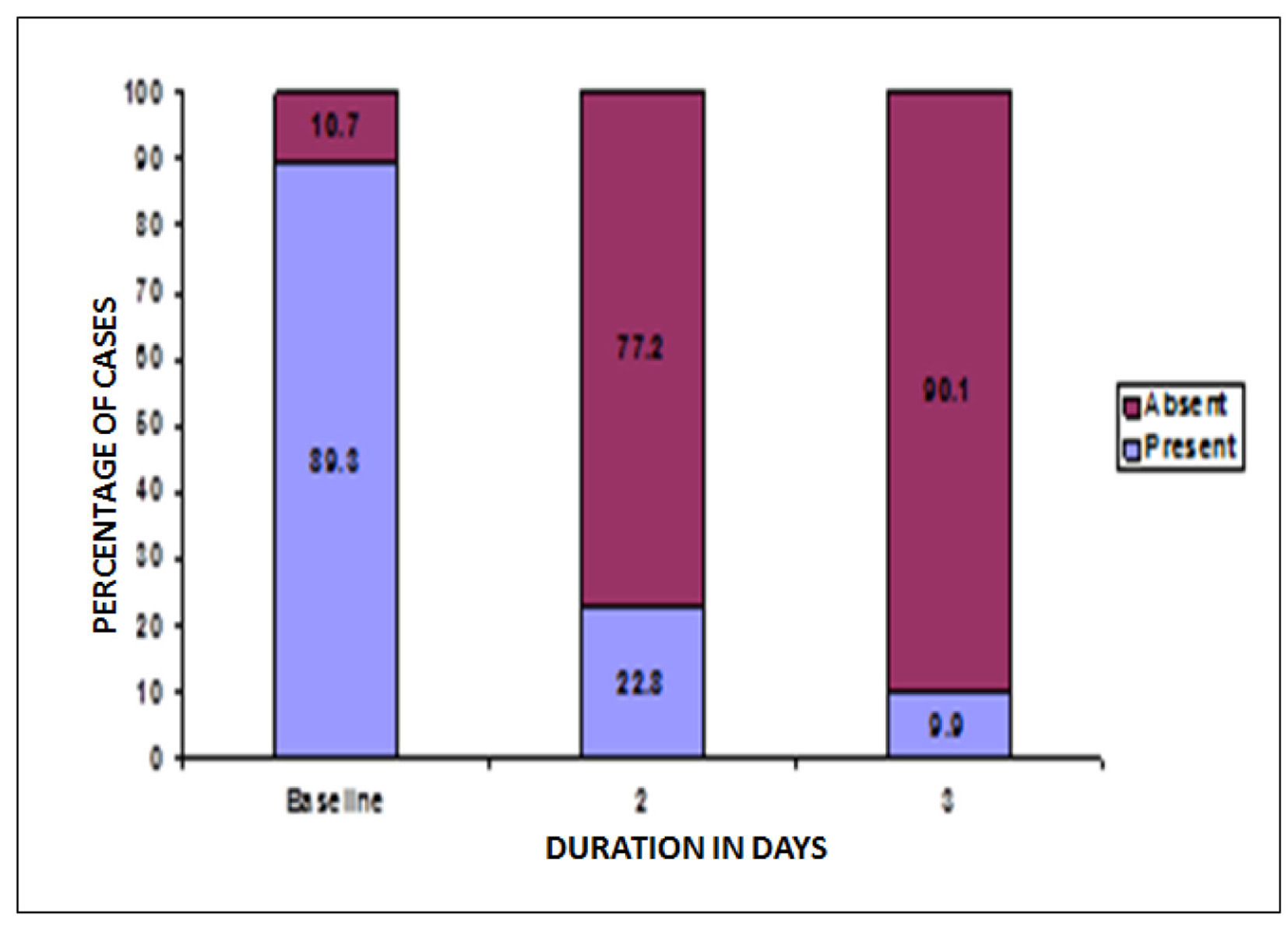 Click for large image | Figure 6. Change in patients with vaginal erythema post-treatment. |
Evaluation of cervical erythema
At baseline, a total of 441 patients reported cervical erythema. Post-treatment, on second and third visit 69.5% and 84.2% females reported absence of cervical erythema. Thus, a statistically significant increase was reported in number of females with no vulvar erythema post-treatment, P < 0.05 (Fig. 7).
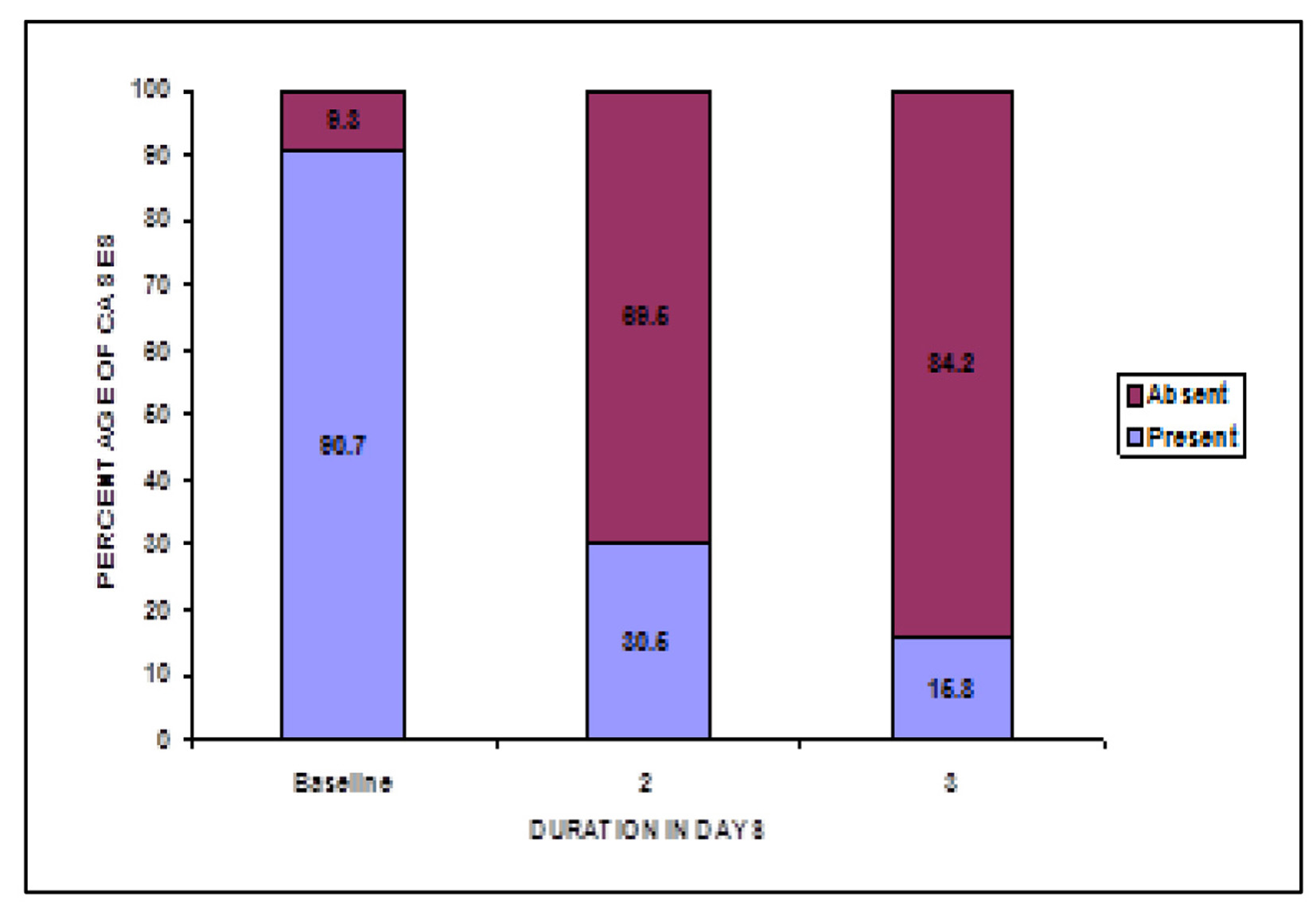 Click for large image | Figure 7. Change in cervical erythema post-treatment. |
Global assessment of efficacy of treatment
As per physician’s evaluation, 85.6% of cases showed very good improvement and 14.4% showed good improvement after treatment (Fig. 8a). As per patients evaluation 86.4% cases showed very good improvement and 13.6% cases showed good improvement after treatment (Fig. 8b).
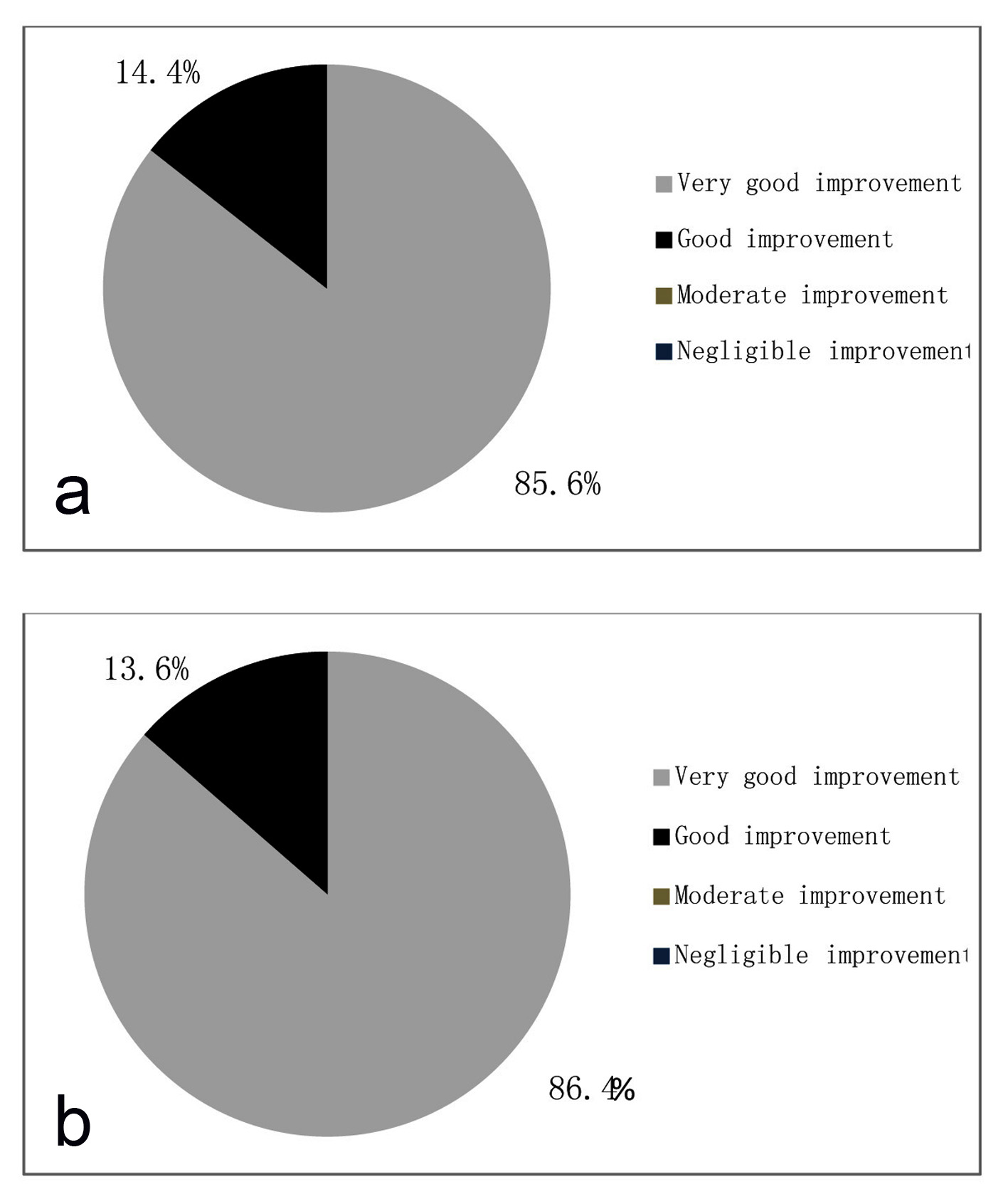 Click for large image | Figure 8. (a) Global assessment of efficacy by physician. (b) Global assessment of efficacy by patient. |
Global assessment of tolerability of treatment
As per physician’s evaluation, 96.1% of cases showed excellent tolerability and 3.9% showed good tolerability after treatment (Fig. 9a). As per patients evaluation 96.3% cases showed excellent tolerability and 3.7% cases showed good tolerability after treatment (Fig. 9b).
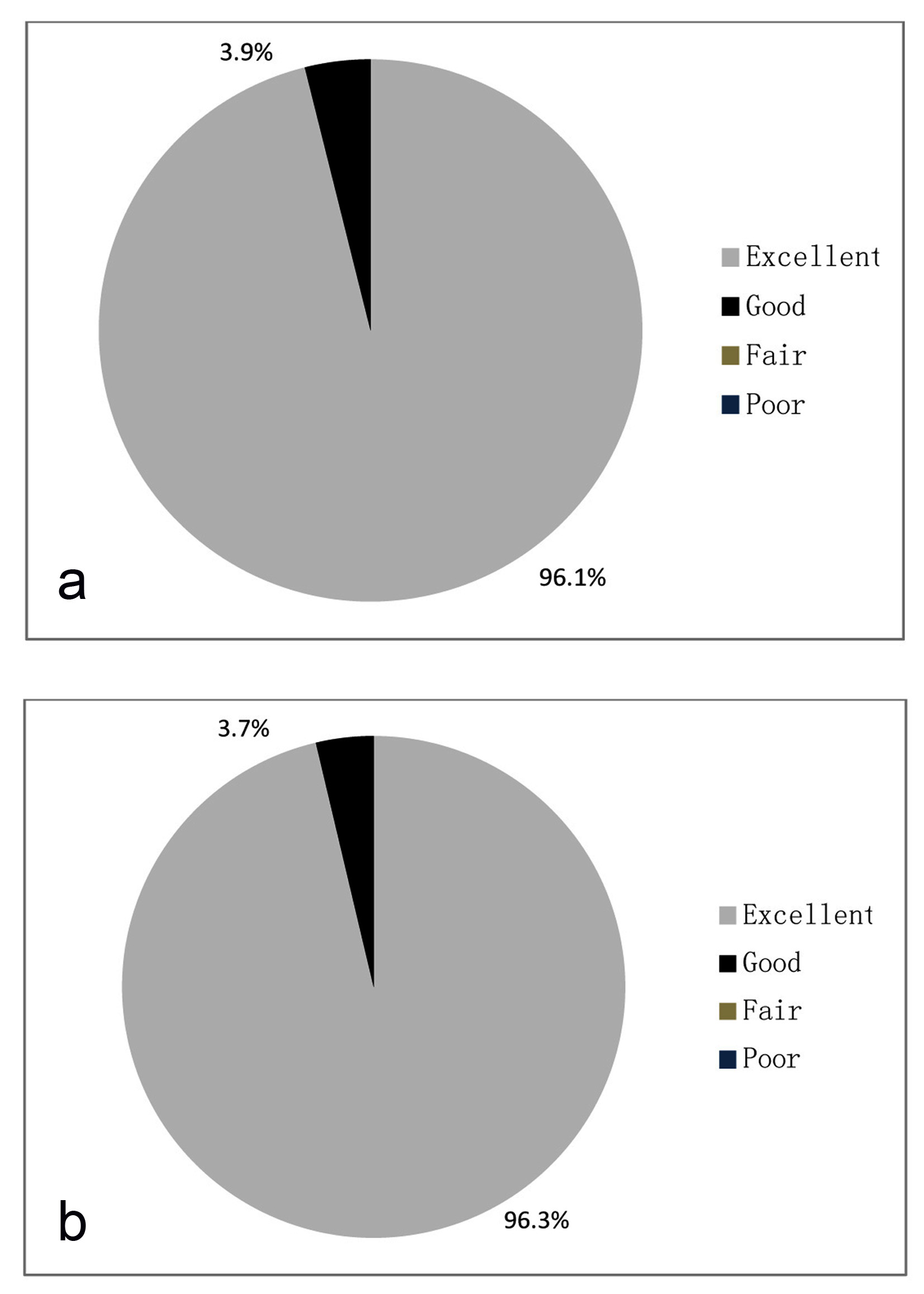 Click for large image | Figure 9. (a) Global assessment of tolerability by physician. (b) Global assessment of tolerability by patient. |
Safety assessment
The present post-marketing surveillance reported 3.7% of the total cases had adverse events. The reported adverse effects were mild to moderate in all the cases. Most of the adverse effects disappeared during treatment and were tolerable. Table 3 enlists adverse events reported during study.
 Click to view | Table 3. Profile of Adverse Events |
| Discussion | ▴Top |
The most common cause of abnormal vaginal discharge is BV, followed by VVC [12].
BV, the most common type of infective vaginosis, is a condition characterized by the partial loss of the indigenous vaginal lactobacilli on the one hand, and massive polymicrobial anerobic overgrowth of the vaginal mucosa on the other [13]. The Amsel criteria are considered to be the standard diagnostic approach to BV and continue to be generally reliable. The criteria are as follows: milky, homogeneous, adherent discharge; vaginal pH greater than 4.5; positive whiff test (the discharge typically has a fishy smell); and presence of clue cells in the vaginal fluid on light microscopy. If three of the four criteria are met, there is a 90% likelihood of BV [14].
Women who harbor candida organisms in their vaginas have VVC, which has a spectrum of manifestations ranging from asymptomatic colonization to severe acute symptomatic infection.
Vaginal candidiasis occurs more commonly after antibiotic treatment and among women taking oral contraceptives [15]. Patients with VVC are usually associated with chief complaints of vulvar pruritis, pain and burning with characteristic thick and white vaginal discharge [16].
Vaginal infections are often polymicrobial; therefore an effective treatment with broad spectrum agents is needed to prevent recurrent relapses. Treatment of BV historically has relied on antibiotics that aim at anerobic and facultative anerobic bacteria. Treatment regimen recommended by the Centre for Disease Prevention and Control (CDC) for BV is either clindamycin or metronidazole [17]. The addition of clotrimazole to clindamycin widens the spectrum of antibacterial action. Clotrimazole a potent antifungal is recommended for the treatment of fungal vaginosis (VVC) [18].
The present post-marketing surveillance study was undertaken to evaluate the safety and efficacy of fixed dose combination of clindamycin and clotrimazole in the treatment of patients with mixed bacterial and fungal vaginosis. The evaluation parameters included vaginal discharge consistency and odor; improvement in vaginal burning and irritation score and change in number of cases reporting vaginal, vulvar and cervical erythema.
Positive clinical responses were seen in majority of treated patients. Clinical assessment showed statistically significant (P < 0.05) improvement with the combination therapy in treatment of troublesome symptoms like vaginal burning and irritation. The severity of vaginal burning and vaginal irritation was reduced by 92.4% and 94.8% respectively within three days of treatment.
The fixed dose combination was effective in treatment of vaginosis and providing relief from the symptoms of infective vaginosis. Beneficial effects and complete eradication of infection was seen after 3 days therapy with clotrimazole and clindamycin.
Following treatment with the combination, a statically significant number of patients showed improvement in clinical indicators of vaginosis. Significant improvement in vaginal discharge was observed in 87.4% of women. The transformation of malodorous vaginal discharge to odorless was seen in 84.8% of women after treatment. More than 92% of the patients reported an improvement in vaginal burning and irritation at the end of the study. Also vulvar, vaginal and cervical erythema disappeared in most of the patients at the end of the study.
The fixed dose combination of clindamycin and clotrimazole combination was well tolerated with relatively few minor side-effects.
Conclusion
Clindamycin and clotrimazole suppository represents a highly effective and safe medication for the treatment of mixed vaginal infection. This combination is effective in providing symptomatic relief in patients with mixed fungal and BV. Also, this combination had good tolerability with no serious side-effects.
| References | ▴Top |
- Ozyurt E, Toykuliyeva MB, Danilyans IL, Morton O, Baktir G. Efficacy of 7-day treatment with metronidazole+miconazole (Neo-Penotran) - a triple-active pessary for the treatment of single and mixed vaginal infections. Int J Gynaecol Obstet. 2001;74(1):35-43.
doi - Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353(18):1899-1911.
doi pubmed - Larsson PG, Fahraeus L, Carlsson B, Jakobsson T, Forsum U. Predisposing factors for bacterial vaginosis, treatment efficacy and pregnancy outcome among term deliveries; results from a preterm delivery study. BMC Womens Health. 2007;7:20.
doi pubmed - Castro J, Henriques A, Machado A, Henriques M, Jefferson KK, Cerca N. Reciprocal interference between Lactobacillus spp. and Gardnerella vaginalis on initial adherence to epithelial cells. Int J Med Sci. 2013;10(9):1193-1198.
doi pubmed - Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23(2):253-273.
doi pubmed - Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369(9577):1961-1971.
doi - Babic M, Hukic M. Candida albicans and non-albicans species as etiological agent of vaginitis in pregnant and non-pregnant women. Bosn J Basic Med Sci. 2010;10(1):89-97.
pubmed - Hainer BL, Gibson MV. Vaginitis. Am Fam Physician. 2011;83(7):807-815.
pubmed - Sobel J, Peipert JF, McGregor JA, Livengood C, Martin M, Robbins J, Wajszczuk CP. Efficacy of clindamycin vaginal ovule (3-day treatment) vs. clindamycin vaginal cream (7-day treatment) in bacterial vaginosis. Infect Dis Obstet Gynecol. 2001;9(1):9-15.
- Spiegel CA. Bacterial vaginosis. Clin Microbiol Rev. 1991;4(4):485-502.
- Graeme Dennerstein. The treatment of Candida vaginitis and vulvitis. Australian Prescriber. 2001;24(3).
- Jeanne M. Marrazzo. Evaluation of Women With Vulvovaginal Complaints. Medscape. Apr 19, 2012.
- Verstraelen H, Verhelst R. Bacterial vaginosis: an update on diagnosis and treatment. Expert Rev Anti Infect Ther. 2009;7(9):1109-1124.
doi pubmed - Owen MK, Clenney TL. Management of vaginitis. Am Fam Physician. 2004;70(11):2125-2132.
pubmed - Nyirjesy P. Vulvovaginal candidiasis and bacterial vaginosis. Infect Dis Clin North Am. 2008;22(4):637-652, vi.
doi pubmed - Eckert LO, Hawes SE, Stevens CE, Koutsky LA, Eschenbach DA, Holmes KK. Vulvovaginal candidiasis: clinical manifestations, risk factors, management algorithm. Obstet Gynecol. 1998;92(5):757-765.
doi - CDC, 2012.
- Zahra Salehei, Zahra S, et al. Sensitivity Vaginal Isolates of Candida to Antifungals. Jundishapur J Microbiol. 2012;5(4):574-577.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.







