| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 5, Number 1, March 2016, pages 17-22
Association Between Endometriosis and Tubo-Ovarian Abscess
Emre Erdem Tasa, Huseyin Levent Keskinb, Gulin Feykan Yegin Akcayb, c, Ulviyye Ismayilovab, Ayse Filiz Yavuza
aDepartment of Gynecology and Obstetrics, Yildirim Beyazit University, Ankara 06830, Turkey
bDepartment of Gynecology and Obstetrics, Ataturk Education and Research Hospital, Ankara 06800, Turkey
cCorresponding Author: Gulin Feykan Yegin Akcay, Ufuk Universitesi Cad. No: 30/30 Cukurambar, Ankara 06610, Turkey
Manuscript accepted for publication February 22, 2016
Short title: Endometriosis and Tubo-Ovarian Abscess
doi: http://dx.doi.org/10.14740/jcgo388w
| Abstract | ▴Top |
Background: Tubo-ovarian abcesses (TOAs) are complicating factors in approximately one-fourth of hospitalized cases of acute pelvic inflammatory disease (PID). However, many women with TOAs have no antecedent history of PID or sexually transmitted diseases. Moreover, it has not been fully elucidated that which risk factors are associated with TOA and TOAs will develop at whom. In recent studies, it has been questioned whether endometriosis is a risk factor for TOA. The purpose of the study was to identify the incidence of endometriosis in patients who have been surgically treated due to TOA and to search clinical-demographical differences among the groups with and without endometriosis.
Methods: A total of 118 patients were divided into two groups according to pathologic results, whether endometriosis with TOAs was confirmed histologically, or not, and demographical-clinical characteristics were investigated.
Results: The mean age of patients was 40.9 ± 8.5 years and 21 (17.8%) were postmenopausal. The intrauterine device was in situ at the time of TOA diagnosis in 49 (41.5%) patients. Concomitant disease and previous pelvic surgery history were identified in 16 (13.6%) and 43 (36.4%) patients, respectively. Final histopathological analyses revealed endometriosis with TOA in 21 (17.8%) patients. When all patients were divided into two groups with and without endometriosis and compared according to clinical and demographical characteristics, there were not any significant differences identified (P > 0.05), except hemoglobin values (P = 0.039).
Conclusion: Endometriosis with TOA is not only in reproductive period but also in menopause, more prevalent than expected for normal population.
Keywords: Endometriosis; Menopause; Surgery; Tubo-ovarian abscess
| Introduction | ▴Top |
Pelvic inflammatory disease or pelvic inflammatory disorder (PID) is an infection of the upper part of the female reproductive system, namely the uterus, fallopian tubes, or the ovaries [1]. The clinical importance of PID is emphasized by its known sequels, which include tubal-factor infertility, ectopic pregnancy and chronic pelvic pain. On the other hand, if the infection is treated inadequately, complications such as pyosalpinx or tubo-ovarian abscesses (TOAs) may occur.
TOAs are polymicrobial infections and similar to PID in terms of risk factors [2]. It has been reported that TOAs are complicating factors in approximately one-fourth of hospitalized cases of acute PID [3]. However, many women with TOAs have no antecedent history of PID or sexually transmitted diseases [4, 5]. Moreover, it has not been fully elucidated that which risk factors are associated with TOA and TOAs will develop at whom.
In recent years, the association between TOAs and endometriosis has been identified and it has been questioned whether endometriosis is a risk factor for TOAs [6, 7]. Therefore, we conducted this retrospective study firstly to investigate the demographical and clinical characteristics of patients who have been surgically treated due to TOAs, secondly to identify the incidence of endometriosis with TOAs, and finally to search for any differences according to clinical characteristics among the groups with and without endometriosis.
| Materials and Methods | ▴Top |
We studied the medical records of women who had been surgically treated (via laparoscopy or laparotomy) for TOAs during a period of 9 years, from January 01, 2006 to December 31, 2014. The study protocol was approved by the Ethics Committee of our university and each patient signed the informed consent.
All patients were treated with one of the following two antibiotic regimens due to the recommended guidelines of Centers for Disease Control (CDC): 1) doxycycline 100 mg intravenously (IV) every 12 h, plus cefoxitin 2 g IV every 6 h or cefotetan 2 g IV every 12 h; 2) clindamycin 900 mg IV 8 h, plus gentamycin 1.5 mg/kg (maintenance dose after initiation with 2 mg/kg) every 8 h and antibiotic therapy were continued IV for at least 7 days [8]. Surgery was performed if there was a failure to respond to appropriate antibiotic therapy within 48 - 72 h (evidence of response includes symptomatic improvement, decreased temperature or leukocyte count, not necessarily a complete cure) or the patient was menopausal.
Data in medical records of 118 patients including age, parity, menopausal status, current intrauterine device (IUD) use, body mass index (BMI, kg/m2), concomitant disease, previous pelvic surgery history, initial complaints, oral body temperature (°C), laboratory values (including C-reactive protein (CRP), cancer antigen 125 (CA 125), white blood cell (WBC) and hemoglobin (Hg)), the mean diameter of abscess (cm) and results of pathological assessment were reviewed. Then, patients were divided into two groups according to pathologic results, whether endometriosis with TOAs was confirmed histologically, or not, and all parameters were compared.
Statistical analyses were performed with SPSS version 17 (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov and Shapiro-Wilk tests were used to assess the data normality. Complementary statistical results were shown as the mean ± standard deviation (SD) for normally distributed data, and as the median (interquartile (IQR)) for non-parametric data. Independent samples t-test, Mann-Whitney U test and Kruskal-Wallis test were used to compare the groups according to distribution of variables. Categorical variables were compared by the Chi-squared test. Statical significance was set at P < 0.0125 for the Bonferroni adjustment test and P < 0.05 for all other tests.
| Results | ▴Top |
A total of 118 patients with a surgical and histological diagnosis of TOA were identified. Among those, 97 (82.2%) were premenopausal and 21 (17.8%) were postmenopausal. The mean age of patients and parity were 40.9 ± 8.5 years and 2.4 ± 1.27, respectively. Furthermore, only six patients (5.1%) were nulliparous. When the patients were admitted to clinic, the most common initial complaints were pelvic pressure (n = 115, 97.5%), abnormal uterine bleeding (n = 15, 12.7%) and fever (n = 9, 7.6%). On the contrary, 28 patients (23.7%) had oral body temperature greater than 38 °C. The mean BMI values were 26.3 (4.1) kg/m2 and BMI values in 61% (n = 77) of patients were greater than 25 kg/m2. In addition, 77 (65.3%) patients were anemic (Hg < 12 g/dL). The clinical characteristics (laboratory values and diameters of abscesses) of all patients are summarized in Table 1.
 Click to view | Table 1. The Clinical Characteristics of Patients With and Without Endometriosis |
Concomitant disease which could have contributed indirectly to abscess formation had accompanied 16 patients (13.6%). Hypertension and diabetes mellitus had constituted approximately three-quarters of such diseases and the distributions of all diseases were displayed with their percents on pie chart (Fig. 1). Previous pelvic surgery history was identified in 43 (36.4%) patients. Most of them, approximately 60%, had previous cesarean sections and three (7%) of the patients were postmenopausal who had undergone total abdominal hysterectomy with bilateral adnexectomy due to leiomyoma, previously. The distributions of all previous pelvic surgeries were displayed with their percents on pie chart, too (Fig. 2).
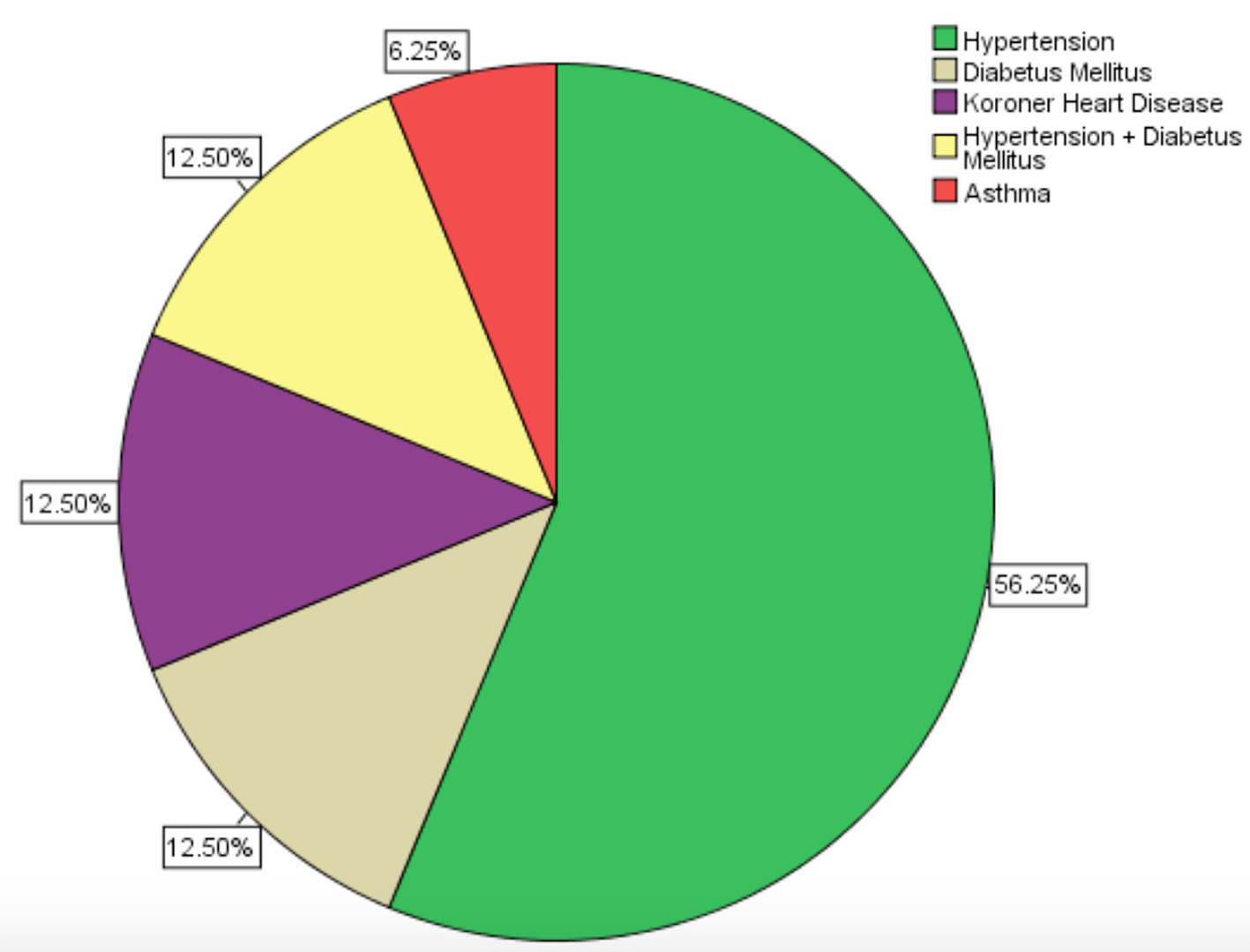 Click for large image | Figure 1. The distributions of the concomitant disease (n = 16). |
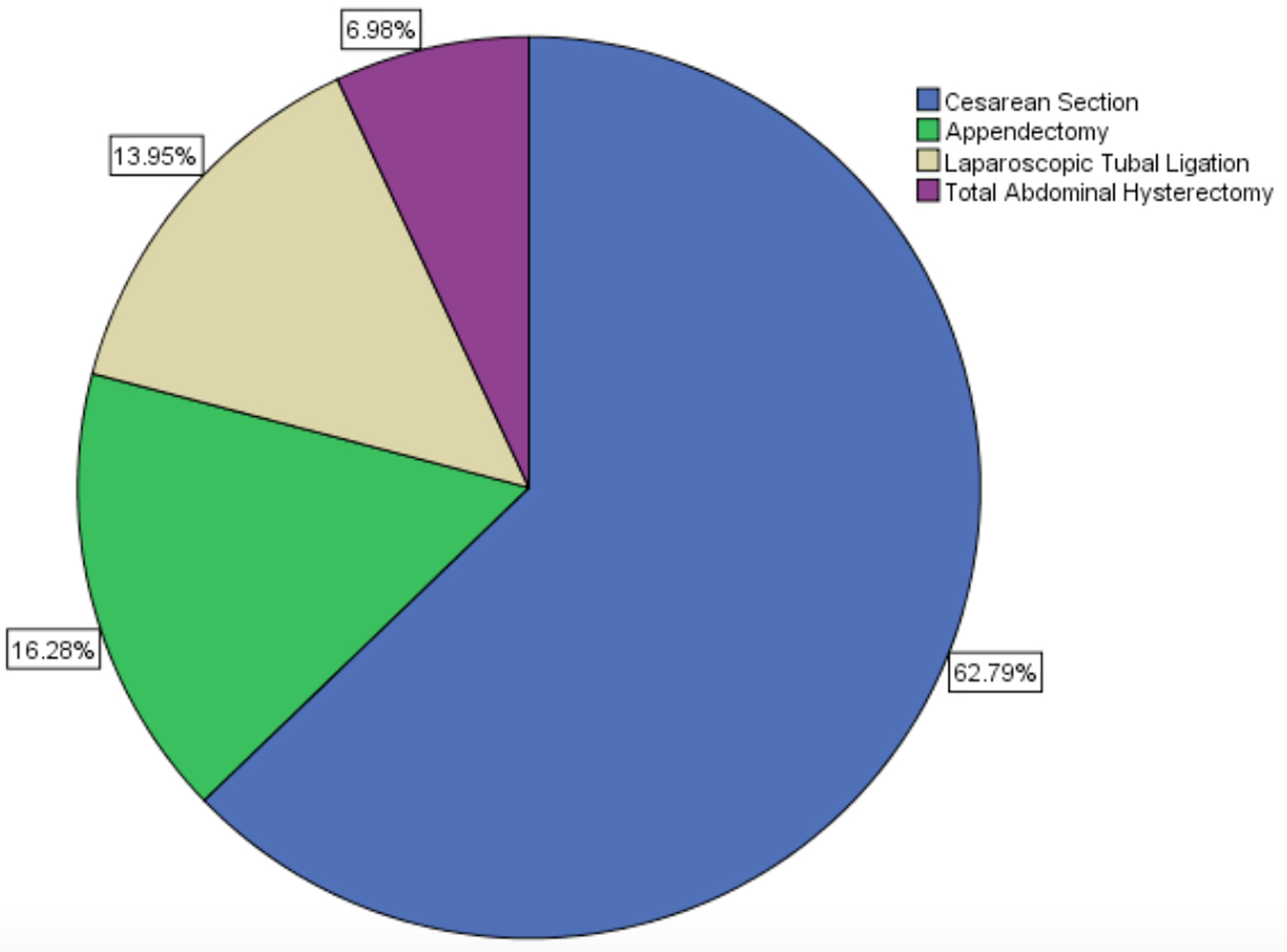 Click for large image | Figure 2. The distributions of the previous pelvic surgery (n = 43). |
The IUD was in situ at the time of TOA diagnosis in 49 (41.5%) patients among all patients and six (5%) patients among the menopausal women. The mean time of interval for women using IUD prior to the diagnosis of TOA was 8.9 (4.02) years. When the patients were divided into two groups according to having IUD or not, Hg, CRP and WBC values were significantly higher in patients with IUD (P values were 0.008, 0.003 and 0.004, respectively) and the clinical characteristics of groups are summarized in Table 2.
 Click to view | Table 2. The Clinical Characteristics of Patients According to Having IUD, or Not |
Hysterectomy (with adnexectomy) was performed in 37 patients (31.4%) via laparotomy and only done to patients whose ages were more than 39 years. However, approximately half of patients (53.2%) whose ages were more than 39 years were treated without hysterectomy. Adnexectomy (without hysterectomy) or cystectomy was performed in the remaining patients (n = 81, 68.6%) via laparoscopy (n = 21, 17.8%) or laparotomy (n = 60, 50.8%). And, when the patients were divided into three groups according to types of operation (hysterectomy with adnexectomy, adnexectomy/cystectomy via laparotomy and adnexectomy/cystectomy via laparoscopy), the mean ages were 47.3 ± 5.7, 37.3 ± 7.9 and 40.1 ± 7.6, respectively. There was a significant difference identified between the groups (P = 0.001) and when the groups were compared within, the mean age was significantly higher in hysterectomy groups than others (P = 0.001). However, there is not any difference identified among the groups who were treated with adnexectomy/drainage via laparotomy or laparoscopy (P = 0.096).
In the result of histopathological analyses, 94 (79.7%) patiens were diagnosed with only TOA formation; however, 21 (17.8%) patients were diagnosed with endometriosis, two (1.7%) patients were diagnosed with teratoma and one (0.8%) patient was diagnosed with benign mucinous ovarian cystadenoma, in addition to TOA. When all patients were divided into two groups with and without endometriosis and compared in terms of clinical characteristics, there were not any significant differences identified between the groups (P > 0.05), except Hg values (P = 0.039) (Table 1). Moreover, when the groups were compared according to having IUD, previous surgery and concomitant disease, there were not any significant differences identified (P values were 0.089, 0.618 and 0.482, respectively).
The mean ages and parities of postmenopausal patients were 51.9 ± 5.3 years and 3.2 ± 1.4, respectively. All the postmenopausal patients were anemic; however, there were not any significant differences identified between pre- and postmenopausal patients in terms of clinical characteristics, except for CA 125 (P = 0.037) (Table 3). Although current IUD users ratio also was higher in premenopausal group (36.4% versus 5.1%), there were not any significant differences identified (P = 0.09). In contrast, concomitant disease ratio was higher in postmenopausal group (33.3% versus 9.3%) and it was significantly higher than premenopausal group (P = 0.009).
 Click to view | Table 3. The Clinical Characteristics of Patients According to Menopausal Status |
Hysterectomy was offered to all patients in menopause; however, it could be performed only to two-thirds of patients (n = 14) because three patients had previous hysterectomy in their histories and four patients had not accepted the proposal of hysterectomy. Final histopathological analyses of excisional materials revealed only TOA formation in 16 (76.2%) and TOA with endometriosis in five (23.8%) patients. When the premenopausal and postmenopausal groups were subdivided into groups with and without endometriosis, and the groups were compared according to histologic results, there were no significant difference identified between the groups (P = 0.528).
| Discussion | ▴Top |
TOAs are generally reported as complicating 10-34% of hospitalized cases of acute PID and approximately 100,000 women are annually hospitalized with TOAs in the United States [3]. With modern broad-spectrum antibiotic therapy, the mortality had also decreased during the last century; however, 12.5-35% of women with PID also require surgery and it remains an important cause of morbidity in young women [8-10].
The initiating event in the formation of a TOA is believed to be the invasion of fallopian tube epithelium by a pathogen, causing tissue damage and necrosis. This damage and necrosis then lead to anaerobic invasion of the fallopian tube and, hence, abscess formation. Although it is not known what the initial factor is responsible for the development of TOAs, some risk factors, such as sexual behavior, increased age, diabetes and immuncompromised status were associated with TOA [4, 7, 11, 12]. Moreover, according to published studies in recent years, endometriosis has been proposed as a risk factor, too [4, 6, 7].
In our study, endometriosis accompanied approximately one-fifth of TOAs and it is more than approximately 4 - 6 times, expected for normal population prevalence [13, 14]. Although we had not classified the patients according to American Society for Reproductive Medicine (ASRM) classification system as Chen et al, the proportion of our patients with endometriosis is half of theirs (17.5% versus 36%). We did not identify any significant differences among the groups in terms of demographic characteristics (age, gravida, and parity) unlike Chen et al; however, we have not confirmed that previous pelvic surgery poses a higher risk of developing TOA in those with endometriosis than in those without endometriosis (P = 0.618), as Chen et al claimed [7].
IUD is the most common method of reversible contraception in the world and it is believed that IUD has been associated with low risk for PID among users [15]. However, a risk for developing TOA has been shown in patients with IUD with period of use exceeding 5 years [16, 17]. Similarly, in our study approximately half of patients with TOAs had IUD and the mean time of interval for women using IUD prior to the diagnosis was 8.9 (4.02) years. Even though some serum inflammatory markers (WBC and CRP) were identified to be significantly higher in patients with IUD users, the effects of IUD on diameter of abscess and erythrocyte sedimentation rate (ESR) have not been identified.
TOAs represent a rather unusual entity in postmenopausal women when compared to those observed in women of reproductive age. The incidence of postmenopausal cases among patients with TOAs had reported between 12% and 18% by researchers previously and was similar to our study (17.8%) [18-20]. In these studies, researchers had reported concomitant genital tract malignancies accompanied by TOAs in 44-47% of postmenopausal women. In contrast, we had not identified any concomitant malignancies in postmenopausal women with TOAs. But we agree with these researchers, and in our clinic, we have offered hysterectomy with adnexectomy to all postmenopausal women with TOAs.
The associations with endometriosis and TOAs have been elucidated previously and several mechanisms have been proposed, e.g. systemic immunologically aberrance, impaired immunity in the pelvic cavity and bloody content of the endometrioma which may serve as a culture medium and facilitate the spread of infection [21, 22]. In this study, we enrolled all the patients with endometriosis and did not classify according to ASRM staging system, because there is no evidence that only advanced stage endometriosis increased the risk of TOA. Moreover, it is too difficult to classify endometriosis in patients with TOAs due to difficulty in distinguishing the lesion origin, e.g. obliteration of the cul-de-sac or adhesions between the pelvic structures.
We addressed that the association between endometriosis and TOAs was not only in reproductive period, but also in menopause (16.5% versus 23.8%, P < 0.05). This condition can be explained with the view that support endometriosis is now considered to be a disease of both endocrine and immune dysregulation [23]. However, we could not identify a significant variable which can predict the endometriosis before initiating therapy with TOAs and thus, prospective studies with new variables are needed.
Conflicts of Interest
The authors have no conflicts of interest relevant to this article.
Financial Disclosures
The authors declare that there is no actual or potential financial interest/arrangement in relation to this article.
| References | ▴Top |
- Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am. 2013;27(4):793-809.
doi pubmed - Washington AE, Aral SO, Wolner-Hanssen P, Grimes DA, Holmes KK. Assessing risk for pelvic inflammatory disease and its sequelae. JAMA. 1991;266(18):2581-2586.
doi pubmed - Landers DV, Sweet RL. Tubo-ovarian abscess: contemporary approach to management. Rev Infect Dis. 1983;5(5):876-884.
doi - Lareau SM, Beigi RH. Pelvic inflammatory disease and tubo-ovarian abscess. Infect Dis Clin North Am. 2008;22(4):693-708, vii.
doi pubmed - Granberg S, Gjelland K, Ekerhovd E. The management of pelvic abscess. Best Pract Res Clin Obstet Gynaecol. 2009;23(5):667-678.
doi pubmed - Kubota T, Ishi K, Takeuchi H. A study of tubo-ovarian and ovarian abscesses, with a focus on cases with endometrioma. J Obstet Gynaecol Res. 1997;23(5):421-426.
doi pubmed - Chen MJ, Yang JH, Yang YS, Ho HN. Increased occurrence of tubo-ovarian abscesses in women with stage III and IV endometriosis. Fertil Steril. 2004;82(2):498-499.
doi pubmed - Walker CK, Wiesenfeld HC. Antibiotic therapy for acute pelvic inflammatory disease: the 2006 Centers for Disease Control and Prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis. 2007;44(Suppl 3):S111-122.
doi pubmed - McNeeley SG, Hendrix SL, Mazzoni MM, Kmak DC, Ransom SB. Medically sound, cost-effective treatment for pelvic inflammatory disease and tuboovarian abscess. Am J Obstet Gynecol. 1998;178(6):1272-1278.
doi - Wiesenfeld HC, Sweet RL. Progress in the management of tuboovarian abscesses. Clin Obstet Gynecol. 1993;36(2):433-444.
doi - Pastorek JG, 2nd. Pelvic inflammatory disease and tubo-ovarian abscess. Obstet Gynecol Clin North Am. 1989;16(2):347-361.
pubmed - Korn AP, Landers DV, Green JR, Sweet RL. Pelvic inflammatory disease in human immunodeficiency virus-infected women. Obstet Gynecol. 1993;82(5):765-768.
pubmed - Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 2002;123(2):217-226.
doi pubmed - Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann N Y Acad Sci. 2002;955:11-22; discussion 34-16, 396-406.
- d'Arcangues C. Worldwide use of intrauterine devices for contraception. Contraception. 2007;75(6 Suppl):S2-7.
doi pubmed - Charonis G, Larsson PG. Prolonged use of intrauterine contraceptive device as a risk factor for tubo-ovarian abscess. Acta Obstet Gynecol Scand. 2009;88(6):680-684.
doi pubmed - Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet. 2000;356(9234):1013-1019.
doi - Protopapas AG, Diakomanolis ES, Milingos SD, Rodolakis AJ, Markaki SN, Vlachos GD, Papadopoulos DE, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114(2):203-209.
doi pubmed - Hsiao SM, Hsieh FJ, Lien YR. Tuboovarian abscesses in postmenopausal women. Taiwan J Obstet Gynecol. 2006;45(3):234-238.
doi - Hoffman M, Molpus K, Roberts WS, Lyman GH, Cavanagh D. Tuboovarian abscess in postmenopausal women. J Reprod Med. 1990;35(5):525-528.
pubmed - Ho HN, Wu MY, Yang YS. Peritoneal cellular immunity and endometriosis. Am J Reprod Immunol. 1997;38(6):400-412.
doi - Padilla SL. Ovarian abscess following puncture of an endometrioma during ultrasound-guided oocyte retrieval. Hum Reprod. 1993;8(8):1282-1283.
pubmed - Kralickova M, Vetvicka V. Immunological aspects of endometriosis: a review. Ann Transl Med. 2015;3(11):153.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.







