| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 5, Number 1, March 2016, pages 27-31
Are Neutrophil/Lymphocyte Ratio and Platelet/Lymphocyte Ratio Predictors for Severity of Preeclampsia?
Mehmet Toptasa, Hilal Asikb, Muhsin Kalyoncuogluc, Esra Cand, Mehmet Mustafa Canc, e
aDepartment of Anesthesiology, Haseki Education and Research Hospital, Fatih, Istanbul, Turkey
bDepartment of Obstetrics and Gynecology, Haseki Education and Research Hospital, Fatih, Istanbul, Turkey
cDepartment of Cardiology, Haseki Education and Research Hospital, Fatih, Istanbul, Turkey
dDepartment of Obstetrics and Gynecology, Kanuni Sultan Suleyman Research and Education Hospital, Fatih, Istanbul, Turkey
eCorresponding Author: Mehmet Mustafa Can, Department of Cardiology, Haseki Education and Research Hospital, Fatih, Istanbul, Turkey
Manuscript accepted for publication March 15, 2016
Short title: Hematologic Indices in Preeclampsia
doi: http://dx.doi.org/10.14740/jcgo389w
| Abstract | ▴Top |
Background: We aimed to assess neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR) levels in patients with preeclampsia (PE) compared with matched normal pregnant women and evaluate whether there is an association of PE severity with hematological paramaters.
Methods: In this study, we recruited 93 women with PE and 94 normal pregnant women matched for both maternal age and gestastional age as controls to assess the clinical, hemodynamic, and neurohormonal status of patients.
Results: There were no statistically significant differences between patients with PE and normal pregnancy with regard to the maternal age and gestation at delivery; however, patients with PE exhibited significantly higher blood pressure, proteinuria levels and parity. Maternal age, multiparity status and the complete blood count parameters including hemoglobin (Hg), white blood cell (WBC), platelets, neutrophil/lymphocyte counts, mean platelet volume (MPV), and mean corpuscular volume (MCV) were not statistically different between the groups. PLR and NLR levels were comparable between PE and normal pregnancies. Moreover, in subgroup analysis, patients with severe PE had similar NLR but lower PLR levels compared to women with mild PE.
Conclusions: PLR was found to be associated with the severity of PE, whereas NLR was not. This finding may be related to cytokine-dependent defective maternal immune activation in PE pathogenesis. In addition, PLR may also be an indirectly available and simple reflector for degree of immune activation in PE.
Keywords: Hematologic indices; Preeclampsia severity
| Introduction | ▴Top |
Preeclampsia (PE) is defined as a new onset of hypertension associated with proteinuria and fluid retention detected for the first time after the 20th week of gestation. PE, or ‘‘toxemia of pregnancy’’ complicates 2-8% of all pregnacies [1].
Although the causes of PE are completely unknown, one of the responsible mechanisms is thought to be activation of inflammatory systems with predominant involvement of cytokines and chemokines [2, 3]. However, there is an ongoing debate about whether inflammatorry system hyperactivity indeed exists during PE, and if available data are sufficient for justification of broad anti-immune system treatment strategies.
Inflammatory statuses of PE have been evaluated using several biomarkers such as C-reactive protein (CRP) and mean platelet volume (MPV). Recently, neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR), which can be derived from the complete blood count (CBC), have been studied as novel markers of prognosis in patients with PE [4, 5]. These studies showed that NLR and PLR could be involved in inflammatory and thrombotic processes. We compared NLR and PLR levels in patients with PE with matched normal pregnant women and evaluated whether there is an association of PE severity with hematological paramaters.
| Methods | ▴Top |
Subjects
The study has been approved by the Local Institutional Review Board and an informed written consent was obtained from each study participant before inclusion. In the current investigation, 93 women with PE were recruited and 94 normal pregnant women matched for both maternal age and gestastional age served as controls. Among the preeclamptic women, 39 were diagnosed with severe PE and 54 mild. PE was diagnosed and classified according to strict criteria recommended by ACOG (2002): a systolic blood pressure of 140 mm Hg or higher or a diastolic blood pressure of 90 mm Hg or higher on two occasions at least 6 h apart occuring after 20 weeks of gestation in a pregnant woman with previously normal blood pressure and detectable urinary protein (>1+ by dipstick or 0.3 g/24 h and more) [6]. Clinical features of patients with severe PE (if any) included a blood pressure greater than or equal to 160/110 mm Hg with either a urine dipstick showing 3+ or 4+ in a random urine sample or greater than 5 g of proteinuria over 24 h. Other evidences of severe disease included serum creatinine, eclampsia, pulmonary edema, oliguria (less than 500 mL/24 h), fetal growth restriction, oligohydramnios and symptoms suggesting significant end-organ involvement (headache and visual disturbance). Women who met the criteria of PE but not severe PE were diagnosed as mild PE. Exclusion criteria were previous lipid metabolism disorders, history of dyslipidemia, multiple gestation, diabetes mellitus, chronic hypertension, infectious diseases diagnosed in pregnancy, premature rupture of membrane, active labor, polyhydramnios, kidney diseases, and signs of other concurrent medical complications. The control subjects had no signs of any gestational complication and fetal distress without evidence of hypertension or proteinuria and all gave health neonates of appropriate size for gestational age.
Sample collection and analysis
The materials were collected immediately after delivery of the fetus in patients and controls whom were in fasting state. Maternal venous was obtained by puncturing the antecubital vein. CBC counts, which included total WBCs, neutrophils, lymphocytes, and platelets, were obtained at the time of admission. The NLR and the PLR were calculated as the ratio of neutrophil count to lymphocyte count and the ratio of platelet count to lymphocyte count, respectively.
Statistical analysis
Continuous variables were expressed as mean ± SD. The level of significance was 0.05. The Kolmogorov-Smirnov test was used for the normality test of all variables. To compare differences between patients with or without disease severity. Mann-Whitney U and Fisher’s exact tests were used for continuous and categorical variables, as appropriate. Statistical analysis was performed using SPSS, version 15.0 for Windows.
| Results | ▴Top |
Clinical and demographic characteristics of our study population are presented in Tables 1 and 2. There were no statistically significant differences between patients with PE and normal pregnancy with regard to the maternal age and gestation at delivery; however, patients with PE exhibited significantly higher blood pressure, proteinuria levels and parity. The characteristics of patients with mild versus severe PE are outlined in Table 3.
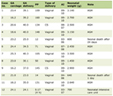 Click to view | Table 1. Baseline Characteristics of Pregnant Women With Preeclampsia and Those Without Preeclampsia |
 Click to view | Table 2. Laboratory Parameters of Pregnant Women With Preeclampsia and Those Without Preeclampsia |
 Click to view | Table 3. Baseline Characteristics of Pregnant Women With Severe and Mild Preeclampsia |
Maternal age, multiparity status and the CBC parameters including hemoglobin (Hg), WBC, platelets, neutrophil/lymphocyte counts, MPV, and MCV were not statistically different between the groups. Fasting glucose, creatinine, TC, LDL cholesterol, HDL cholesterol, TG, GGT and uric acid levels were similar between the two groups. PLR and NLR levels were comparable between PE and normal pregnancies. Moreover, in subgroup analysis, patients with severe PE had similar NLR but lower PLR levels compared to women with mild PE.
| Discussion | ▴Top |
PE is a multisystem disorder that leads to end-organ damage and/or hypoperfusion such as renal dysfunction (proteinuria, increased creatinine level or glomerular endotheliosis), hematological dysfunction (hemolysis, disseminated intravascular coagulation, and thrombocytopenia), hepatic dysfunction (raised transaminases), and neurological dysfunction (hyperreflexia and visual disturbances) [7, 8]. In the present study, we observed end-organ damage was higher in patients with severe PE and/or fetal growth restriction than mild preeclamptic and normal pregnants in accordance with data.
Although the exact etiology is unknown, many theories suggested that abnormal placentation leading to inflammation in microvasculature and releasing of microparticles and anti-angiogenic factors into the maternal systemic circulation, is one of the initial events in the pathogenesis of PE. The activation of the physiological inflammatory pathway that happens in normal pregnancy is activated excessively in PE. Increased soluble factors initiate activation of platelets, production of inflammatory cytokines, and vascular endothelial dysfunction [7-9]. Besides that, activating platelets release various soluble and adhesion molecules which trigger the interactions between platelets, leukocytes, and endothelial cells. Pevious data suggest that platelets play a major role in the pathogenesis of PE [10-12]. There are conflicting data including relation on blood cells counts and development and/or severity of PE [10, 13-16]. In the present study, platelet, leukocyte and lymphocyte counts did not exhibit significant difference in normal pregnant, severe and mild preeclamptic patients in accordance with some of previous reports [10, 13]. MPV is a parameter providing information on platelet activity and can increase or decrease depending on the severity of the inflammatory response [12]. There are controversial data about association of MPV and PE. In contrast to the current study, both Yavuzcan et al and Ceyhan et al indicated that MPV did not change in severe PE, whereas some previous data stated that it is possible to estimate disease severity on basis of MPV measurement [10, 12, 14, 17, 18]. In our study, although we found that MPV was higher in preeclamptic patients than normal, we did not observe any relation between the MPV and the severity of PE. So, the data on the significance of CBC parameters in the literature are contradictory. When considering the previous reports and the present study, we assumed that CBC parameters could not be used as an isolated factor to determine the severity of PE.
As far as we know, this is the first report in the literature to evaluate the association between the severity of PE and NLR and PLR in preeclamptic patients. NLR and PLR obtained from CBC in peripheral blood are used to be non-specific markers of systemic inflammatory response (SIR) [19]. NLR denotes the ratio of neutrophils, representing the active non-specific inflammatory mediator initiating the first line of defense, to lymphocytes representing the regulatory or protective component of inflammation [20]. Previous numerous studies showed that NLR has prognostic and predictive value in various benign and malignant diseases such as coronary artery disease, inflammatory diseases, PE, and gynecologic and gastrointestinal malignancies [14, 19, 21]. In the present study, NLR was significantly higher in patients with PE than normal pregnancies, but did not exhibit significantly difference between the patients with severe and mild PE. Similar to our study, Oylumlu et al demonstrated that increased levels of NLR were independently associated in patients with PE compared to healthy pregnancies [22]. In another study, in contrast to our findings, Yavuzcan et al reported that NLR was not meaningfully differrent in patients with severe PE and healthy pregnant women [14]. In the light of the literature and the present study, although NLR has been proposed as a new indicator of SIR and its predictive and prognostic values in many different pathologies have been demonstrated in the previous studies, we assumed that NLR may be a predictor for existing disease but not for severity of SIR in PE.
The maternal immune system plays an important role in both the placentation and the subsequent systemic reactions [23, 24]. There is still no exactly defined etiology of PE, but it is proposed that an excessive maternal SIR with activation of both the innate and adaptive arms of the immune system is an integral part of the pathogenesis [25, 26]. In contrast to normal pregnancy, systemic inflammation in PE has the predominancy of Th1-type immunity than the Th2 tendency and includes the cellular immunity with an increase of pro-inflammatory and a decrease of anti-inflammatory cytokines [27, 28]. Platelets and lymphocytes are significant blood parameters related to immune surveillance and the PLR plays an important role in cytokine-dependent immune response [29, 30]. It was proposed that PLR was a more sensitive marker of systemic inflammation and prognostic factor in breast cancer, ovarian and colorectal cancers [19]. Although association of PLR with inflammation and numerous diseases was reported in the literature, there are limitted data evaluating the association of PLR and the PE [14, 19, 31]. Similar to our findings, Yavuzcan et al demonstrated that PLR was not significantly different between the patients with severe PE and healthy pregnancies [14]. Although we did not observe any difference between the preeclamptic patients and the healthy pregnancies, we demonstrated significantly higher PLR in severe PE than mild. So, in the current study, we observed that PLR was predictive for severity of PE, whereas NLR was not. In the light of current information obtained form the literature and the present study, we assume that PLR is an easily available and cost-efective reflector of activated cytokine-dependent maternal immune system and predictor of severity of PE, whereas NLR may be a reflector for the existing disease but not for severity of disease.
The main limitation of the current study was the small study population. In the study we only determined the ratio of platelet, neutrophil and total lymphocyte counts without regarding the T lymphocyte subtypes indicating immune activation and the relation of these subtypes with the others such as N/Th1, N/Th2, P/Th1 and P/Th2.
Conclusion
In the present study, PLR was found to be associated with the severity of PE, whereas NLR was not. This finding may be related to cytokine-dependent defective maternal immune activation in PE pathogenesis. In addition, PLR may also be an indirectly available and simple reflector for degree of immune activation in PE. But the data about the importance of PLR and NLR in PE are insufficient and further research is required to elucidate the significance of PRL and NLR in PE.
Conflict of Interest
No conflict of interest regarding the research, authorship, and/or publication of this article was declared.
Funding
No external funding was provided for this study.
| References | ▴Top |
- Turner JA. Diagnosis and management of pre-eclampsia: an update. Int J Womens Health. 2010;2:327-337.
doi pubmed - Reslan OM, Khalil RA. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc Hematol Agents Med Chem. 2010;8(4):204-226.
doi - Lamarca B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva Ginecol. 2010;62(2):105-120.
pubmed - Dogan K, Guraslan H, Senturk MB, Helvacioglu C, Idil S, Ekin M. Can Platelet Count and Platelet Indices Predict the Risk and the Prognosis of Preeclampsia? Hypertens Pregnancy. 2015;34(4):434-442.
doi pubmed - Han L, Liu X, Li H, Zou J, Yang Z, Han J, Huang W, et al. Blood coagulation parameters and platelet indices: changes in normal and preeclamptic pregnancies and predictive values for preeclampsia. PLoS One. 2014;9(12):e114488.
doi pubmed - ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;77(1):67-75.
pubmed - Hladunewich M, Karumanchi SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol. 2007;2(3):543-549.
doi pubmed - Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63(18):1815-1822.
doi pubmed - Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631-644.
doi - Ceyhan T, Beyan C, Baser I, Kaptan K, Gungor S, Ifran A. The effect of pre-eclampsia on complete blood count, platelet count and mean platelet volume. Ann Hematol. 2006;85(5):320-322.
doi pubmed - Sadik Sahin S, Ozakpinar OB, Eroglu M, Tetik S. Platelets in Preeclampsia: Function and Role in the Inflammation. MUSBED. 2014;4(2):111-116.
- Karateke A, Kurt RK, Baloglu A. Relation of platelet distribution width (PDW) and platelet crit (PCT) to preeclampsia. Ginekol Pol. 2015;86(5):372-375.
doi pubmed - Neiger R, Contag SA, Coustan DR. Preeclampsia effect on platelet count. Am J Perinatol. 1992;9(5-6):378-380.
doi pubmed - Yavuzcan A, Caglar M, Ustun Y, Dilbaz S, Ozdemir I, Yildiz E, Ozbilgec S, et al. Mean platelet volume, neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in severe preeclampsia. Ginekol Pol. 2014;85(3):197-203.
doi - Canzoneri BJ, Lewis DF, Groome L, Wang Y. Increased neutrophil numbers account for leukocytosis in women with preeclampsia. Am J Perinatol. 2009;26(10):729-732.
doi pubmed - Lurie S, Frenkel E, Tuvbin Y. Comparison of the differential distribution of leukocytes in preeclampsia versus uncomplicated pregnancy. Gynecol Obstet Invest. 1998;45(4):229-231.
doi pubmed - Dundar O, Yoruk P, Tutuncu L, Erikci AA, Muhcu M, Ergur AR, Atay V, et al. Longitudinal study of platelet size changes in gestation and predictive power of elevated MPV in development of pre-eclampsia. Prenat Diagn. 2008;28(11):1052-1056.
doi pubmed - Jaremo P, Lindahl TL, Lennmarken C, Forsgren H. The use of platelet density and volume measurements to estimate the severity of pre-eclampsia. Eur J Clin Invest. 2000;30(12):1113-1118.
doi pubmed - Cakmak B, Gulucu S, Aliyev N, Ozsoy Z, Nacar M, Koseoglu D. Neutrophil-lymphocyte and platelet-lymphocyte ratios in endometrial hyperplasia. Obstet Gynecol Sci. 2015;58(2):157-161.
doi pubmed - Bhutta H, Agha R, Wong J, Tang TY, Wilson YG, Walsh SR. Neutrophillymphocyte ratio predicts medium-term survival following elective major vascular surgery: a crosssectional study. Vasc Endovascular Surg. 2011;45:227-231.
doi pubmed - Kurtoglu E, Kokcu A, Celik H, Sari S, Tosun M. Platelet Indices May be Useful in Discrimination of Benign and Malign Endometrial Lesions, and Early and Advanced Stage Endometrial Cancer. Asian Pac J Cancer Prev. 2015;16(13):5397-5400.
doi pubmed - Oylumlu M, Ozler A, Yildiz A, Acet H, Polat N, Soydinc HE, Yuksel M, et al. New inflammatory markers in pre-eclampsia: echocardiographic epicardial fat thickness and neutrophil to lymphocyte ratio. Clin Exp Hypertens. 2014;36(7):503-507.
doi pubmed - Jonsson Y, Ekerfelt C, Berg G, Nieminen K, Sharma S, Ernerudh J, Matthiesen L. Systemic Th1/Th2 cytokine responses to paternal and vaccination antigens in preeclampsia: no differences compared with normal pregnancy. Am J Reprod Immunol. 2004;51(4):302-310.
doi pubmed - Jonsson Y, Matthiesen L, Berg G, Ernerudh J, Nieminen K, Ekerfelt C. Indications of an altered immune balance in preeclampsia: a decrease in in vitro secretion of IL-5 and IL-10 from blood mononuclear cells and in blood basophil counts compared with normal pregnancy. J Reprod Immunol. 2005;66(1):69-84.
doi pubmed - Molvarec A, Shiozaki A, Ito M, Toldi G, Stenczer B, Szarka A, Nakashima A, et al. Increased prevalence of peripheral blood granulysin-producing cytotoxic T lymphocytes in preeclampsia. J Reprod Immunol. 2011;91(1-2):56-63.
doi pubmed - Matthiesen L, Berg G, Ernerudh J, Ekerfelt C, Jonsson Y, Sharma S. Immunology of preeclampsia. Chem Immunol Allergy. 2005;89:49-61.
doi pubmed - Ramma W, Ahmed A. Is inflammation the cause of pre-eclampsia? Biochem Soc Trans. 2011;39(6):1619-1627.
doi pubmed - Boij R, Svensson J, Nilsson-Ekdahl K, Sandholm K, Lindahl TL, Palonek E, Garle M, et al. Biomarkers of coagulation, inflammation, and angiogenesis are independently associated with preeclampsia. Am J Reprod Immunol. 2012;68(3):258-270.
doi pubmed - Tasoglu I, Sert D, Colak N, Uzun A, Songur M, Ecevit A. Neutrophil-lymphocyte ratio and the platelet-lymphocyte ratio predict the limb survival in critical limb ischemia. Clin Appl Thromb Hemost. 2014;20(6):645-650.
doi pubmed - Turkmen K, Erdur FM, Ozcicek F, Ozcicek A, Akbas EM, Ozbicer A, Demirtas L, et al. Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodial Int. 2013;17(3):391-396.
doi pubmed - Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O'Reilly DS, Foulis AK, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633-2641.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.







