| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 5, Number 3, September 2016, pages 85-91
Mutations in the Gene for Factor V Leiden and G20210A Prothrombin Polymorphism in Women With Recurrent Spontaneous Abortion: A Retrospective Study in a Brazilian Population
Mariana Maaz Proitea, Andrea Cristina de Moraes Malinvernia, e, Celina Tizuko Fujiyama Oshimaa, Marcia Marcelino de Souza Ishigaib, Antonio Fernandes Moronc, Ismael Dale Cotrin Guerreiro da Silvad, Francy Reis da Silvab
aMolecular Pathology Laboratory, Department of Pathology, Universidade Federal de Sao Paulo (UNIFESP), Sao Paulo, Brazil
bPediatric Discipline, Department of Pathology, Universidade Federal de Sao Paulo (UNIFESP), Sao Paulo, Brazil
cFetal Medicine Division, Department of Obstetrics, Universidade Federal de Sao Paulo (UNIFESP), Sao Paulo, Brazil
dMolecular Laboratory, Department of Gynecology, Universidade Federal de Sao Paulo (UNIFESP), Sao Paulo, Brazil
eCorresponding Author: Andrea Cristina de Moraes Malinverni, Molecular Pathology Laboratory, Department of Pathology, Universidade Federal de Sao Paulo (UNIFESP), Sao Paulo, Brazil, Rua Pedro de Toledo, 781, 5º andar, Vila Clementino, CEP: 04039-032, Sao Paulo, SP, Brazil
Manuscript accepted for publication July 05, 2016
Short title: Mutations of Gene FVL in Women
doi: http://dx.doi.org/10.14740/jcgo412e
| Abstract | ▴Top |
Background: Pregnancy is sufficiently considered an important risk factor for thromboembolic events. We aimed to determine the prevalence of factor V Leiden (FVL) and prothrombin gene 20210A mutations in patients with miscarriages and to correlate these results with normal and abnormal karyotypes.
Methods: A retrospective study was carried out in 247 women with a history of miscarriages. These samples were karyotyped, and the DNA was extracted (chorionic villi) and genotyped for FVL and prothrombin gene polymorphism.
Results: Polymorphism frequency was compared among different groups. FLV showed significant levels in the normal cytogenetic groups in two comparisons: normal abortion (NA) versus abnormal abortion (AA) (P = 0.08) and normal recurrent abortion (NRA) versus AA (P = 0.048). The presence or absence of at least one polymorphism was evaluated. The frequency of G20210A prothrombin gene polymorphism was higher in NA than in AA: NA versus AA (P = 0.014), NRA versus AA (P = 0.018) and AA versus NRA (P = 0.01).
Conclusions: The FVL showed probable importance in the genesis of abortions due to its greater frequency of normal karyotype miscarriage. Once presence of both polymorphisms was significantly higher in normal karyotype miscarriage than in abnormal, we can infer that the alterations are associated with the pathophysiology of abortions.
Keywords: Factor V Leiden; Prothrombin gene polymorphism; Recurrent spontaneous abortion; Molecular alteration; Karyotype
| Introduction | ▴Top |
Miscarriage is a common event in medicine, affecting about 1-2% of women of reproductive age [1]. It is estimated that 15-25% of pregnancies culminate in abortion, and up to 5% are recurrent [2, 3]. The habitual spontaneous abortion (HSA) or recurrent spontaneous abortion (RSA) can be defined as the loss of three or more consecutive miscarriages before the 20th week of pregnancy. Chromosomal abnormalities account for more than 50% of the causes of abortions, especially in the first quarter of pregnancy [4-6]. Other anomalies, such as the reproductive system, hormonal disorders, immune factors, autoimmune diseases and infectious diseases are also associated with the genesis of abortions [7] and still 30-50% of the cases remain unexplained [8]. The adaptation of hemostasis during pregnancy induces a tendency for placental thrombosis. The pregnancy is sufficiently considered an important risk factor for thromboembolic events and the presence of other factors during pregnancy can greatly increase the risk. Among the inherited thrombophilia, there is resistance to C-reactive protein, mainly caused by the presence of factor V Leiden (FVL) in about 95% of cases. The FVL gene may undergo a point mutation at nucleotide 1691 (G→A) OMIM #227400. This leads to substitution of arginine for glutamine at position 506 and is located on the long arm of chromosome 1 (1q23). The FVL mutation makes the patient with FV coagulation be resistant to down-regulation by activated protein C. The major cause of venous thrombosis is associated with patients who have a history of oral contraceptive use. The FVL has been found in about 20-40% of these patients and in 60% of patients during pregnancy [6, 9, 10]. Moreover, high prothrombin levels in the plasma and increased risk of arterial and venous thrombosis can be associated to polymorphisms in 3'-UT region of the prothrombin gene (the G→A substitution at nucleotide position 20210) [11, 12]. According to the problem presented, the purpose of this study was to evaluate the presence of mutation in the FVL and in G20210A prothrombin gene polymorphism, correlating the possible findings with miscarriages and previous cytogenetic results.
| Materials and Methods | ▴Top |
A retrospective study was carried out in 247 women with a history of miscarriages. These samples were sent to cytogenetic analyse, during the year 2004. This study was approved by a research Ethics Committee of the Federal University of Sao Paulo/Paulista School of Medicine (CEP 1523/04).
Cytogenetic analysis
Chorionic villi sample of placental or placental fragments were sent between the fifth and 20th gestational week in sterile flask containing 10 mL of sterile saline solution. The time between collecting material and sending to the laboratory did not exceed 48 h, with the material stored refrigerated (4 - 8 °C). This material was analyzed microscopically to choose the adequate sample for the cell culture (chorionic villi sample). Until the 13th week of pregnancy, semi-direct culture was performed and above the 14th week, long-term culture was used according to the protocol pre-established by the laboratory and literature [13-16]. After the culture had been performed by harvesting methods and slide staining with Band G according to laboratory protocols, the karyotypes were analyzed according to International System for Human Cytogenetic Nomenclature 2005 (ISCN) standards.
Molecular biology
The extraction of DNA from chorionic villi, as well as the polymerase chain reactions (PCRs) and enzymatic digestions were performed according to the standardization of the Molecular Biology Laboratory of the Department of Gynecology of the School of Medicine Paulista, UNIFESP. The chorionic villi were placed in sterile bottle medium 199 Cultilab® and stored in the freezer at -20 °C until DNA extraction. Miniprep System GIBCO® was used for DNA extraction. In some samples with impurities, it was necessary to realize the purification of proteins using the GFX® kit from Amersham-Pharmacia. DNAs were stored at -20 °C until PCR analysis.
Genotyping of FVL
Amplification of human DNA genomic sequences with polymerase chain reaction (PCR) using sequences of the oligonucleotides primer had been described earlier by Eiben et al (1994) (5’-TGTTATCACACTGGCGCTAA3’ and 3’-GAACAATTCGTGACCCGT-5’) and was used in this study. The PCR was used to amplify the exon 10 of the FVL gene; when the mutation occurred, there was the G→A transition at nucleotide 1691. Amplification was carried out in a final volume of 25 μL containing 0.5 μL of each primer (10 pmol/μL), 2 μL of genomic DNA (50 - 200 ng), 11 μL PCR Master Mix (Promega 50 U/mL Taq polymerase, 400 μM dNTP, 3 mM MgCl2) and 11 μL sterile water. For all reactions, a negative control was used, where all reactants except DNA (to avoid contamination) were placed into 1.5 mL sterile Eppendorf tubes. PCR was processed in an Eppendorf thermocycler and the reaction was denatured at 94 °C for 4 min followed by 40 cycles of 94 °C (60 s), 55 °C (60 s) and 72 °C (60 s), and after these cycles, 72 °C (7 min) and 4 °C until turn off the equipment. The PCR products amplified were visualized on 2% agarose gel stained with ethidium bromide. The product amplified was 267 pair (bp) and to enzymatic digestion, a specific restriction enzyme (Mnl1, New England Bio Labs®) was used for mutation detection. The digestion product was revealed on 3% agarose gel, and stained with ethidium bromide and the gel was photographed in Kodak® system. In both electrophoreses, molecular weight markers (100 pb, Invitrogen) were run to verify if the bands corresponded to molecular weight, even if some bands were run off the bottom of the gel.
Genotyping factor II/prothrombin G20210A
The prothrombin gene region was amplified by PCR in the presence of the primers sequences described by Poort et al (1996): 5’-ATAGCACTGGGAGCATTGAAGC-3’ and 3’-CGGTCCGTTGACAAAGATCT-5’. The PCRs were performed under the same conditions of factor V, including the number of cycles. Only the second temperature in the cycle was different. We change 55 °C to 58 °C. Endonuclease HindIII was used as specific restriction enzyme (New England Bio Labs®).
Statistical analysis
The Hardy-Weinberg, allelic and genotypic frequencies were compared using the Chi-square test or Fisher’s exact test, when < 5 were observed in at least one category, which they were being compared. The odds ratio were derived from logistic regression model using the SPSS statistical package (Statiscal Package for Social Science, v12.0). For analysis of quantitative variables, we used the analysis of variance (ANOVA) for normal data distribution, but Mann-Whitney test when the assumption of normal distribution of data was rejected.
| Results | ▴Top |
In the 247 samples analyzed, 210 had cell growth and of these, 123 chromosomal abnormalities, 75 had habitual abortion and 48 had isolated abortion. Table 1 shows the frequencies of chromosomal abnormalities found in these samples, which among the aneuploidy, we had 74% trisomy, 14.6% euploidy, 3.25% tetraploidy, 3.25 double trisomy, 1.65% monosomy and 3.25% of structural abnormalities. For FVL molecular analysis, 247 abortion samples were submitted, of which 11 (4.45%) were not amplified and 236 (95.55%) were amplified. Among these samples, 224 (94.02%) were homozygous wild and 12 (5.08%) were heterozygous. Homozygous genotype not mutated for this polymorphism was found. Figure 1 shows a 3% agarose gel with the results for FVL. The presence of three bands of 116, 67 and 37 pb showed the wild-type homozygous genotype (without the mutation on both alleles) and the presence of four fragments of 153, 116, 67 and 37 pb characterized heterozygous genotype (one mutated allele and one wild). The same samples were also submitted to study G20210A prothrombin gene polymorphism, of which 20 (8.09%) were not amplified and 221 (97.36%) were amplified and homozygous wild and six (2.64%) were heterozygous. The homozygous genotype mutated to this polymorphism was not found. Figure 2 shows a 3% agarose gel for the polymorphism of the prothrombin gene. The band of 345 pb was the wild homozygous genotype and two bands 345 pb and 322 pb were the heterozygous genotype. Both polymorphisms analyzed showed in the sample total in Hardy-Weinberg equilibrium: X2 = 0.16, P > 0.05 and X2 = 0.04, P > 0.05 for the FVL and prothrombin polymorphism, respectively. There was no statistically significant difference between habitual abortion (HA) and isolated abortion (IA); normal isolated abortion (NIA) and abnormal isolated abortion (AIA); abnormal habitual abortion (AHA) and NIA; AHA and AIA; abnormal abortion (AA) and NIA. Regarding prothrombin analysis, statistically significant differences were observed between the groups: AA and IA; NIA and AIA; normal habitual abortion (NHA) and NIA; AHA and AIA; AA and NHA; AA and NIA; HA and IA. An analysis was also performed considering the presence or absence of at least one of the polymorphisms studied. When HA and IA groups were compared to the polymorphism of FV, although a level of significance was not reached, there was a tendency for a positive association between the group of normal abortions and FVL polymorphism present in heterozygous (Fisher’s exact test, P = 0.08, odds ratio: 0.305, 95% CI: 0.08 - 1.156). The comparison between the presence and absence of at least one of the polymorphisms study found a statistically significant result (Fisher’s exact test, P = 0.014; odds ratio: 3.921, 95% CI: 12.5 - 1.251*). Therefore, the frequency of at least one of the polymorphisms in normal abortion (NA) group was significantly higher, compared to the frequency of at least one of the polymorphisms in the AA group. NA was about four times more likely to have at least one of the polymorphisms compared to AA. When the presence of at least one of the polymorphisms was taken into account, a statistically significant result was obtained (Fisher’s exact test, P = 0.018; odds ratio: 5.847, 95% CI: 38.571 - 1.246). Thus, it can be noticed that the presence of a mutated allele is significantly higher in NHA group compared to AHA group. NHA was about six times more likely to have one of the polymorphisms than AHA. Abnormal miscarriages and abortions normal usual: when comparing the frequency of FVL between AA and NHA, we found a close difference to the level of significance (Fisher’s exact test, P = 0.048; odds ratio: 3.95; 95% CI: 0.99 - 15.8), suggesting that about the probability of having this polymorphism, NHA was approximately four times higher compared with the AA. Concomitantly, the polymorphism analysis showed a statistically significant difference (Fisher’s exact test, P = 0.01, odds ratio: 4.87; 95% CI: 1.49 - 15.87), thus, NHAs were about five times more likely to have one of the polymorphisms compared with altered abortions. AAs and NIAs (Table 2): the analysis of the presence or absence of at least one of the polymorphisms did not show statistically significant difference between AA and NIA (Fisher’s exact test, P = 0.372; odds ratio: 0.436, 95% CI: 0.093 - 2.043). A comparison of the frequency of at least one of the polymorphisms did not show difference between groups.
 Click to view | Table 1. Cytogenetic Results and Frequencies of Chromosomal Abnormalities |
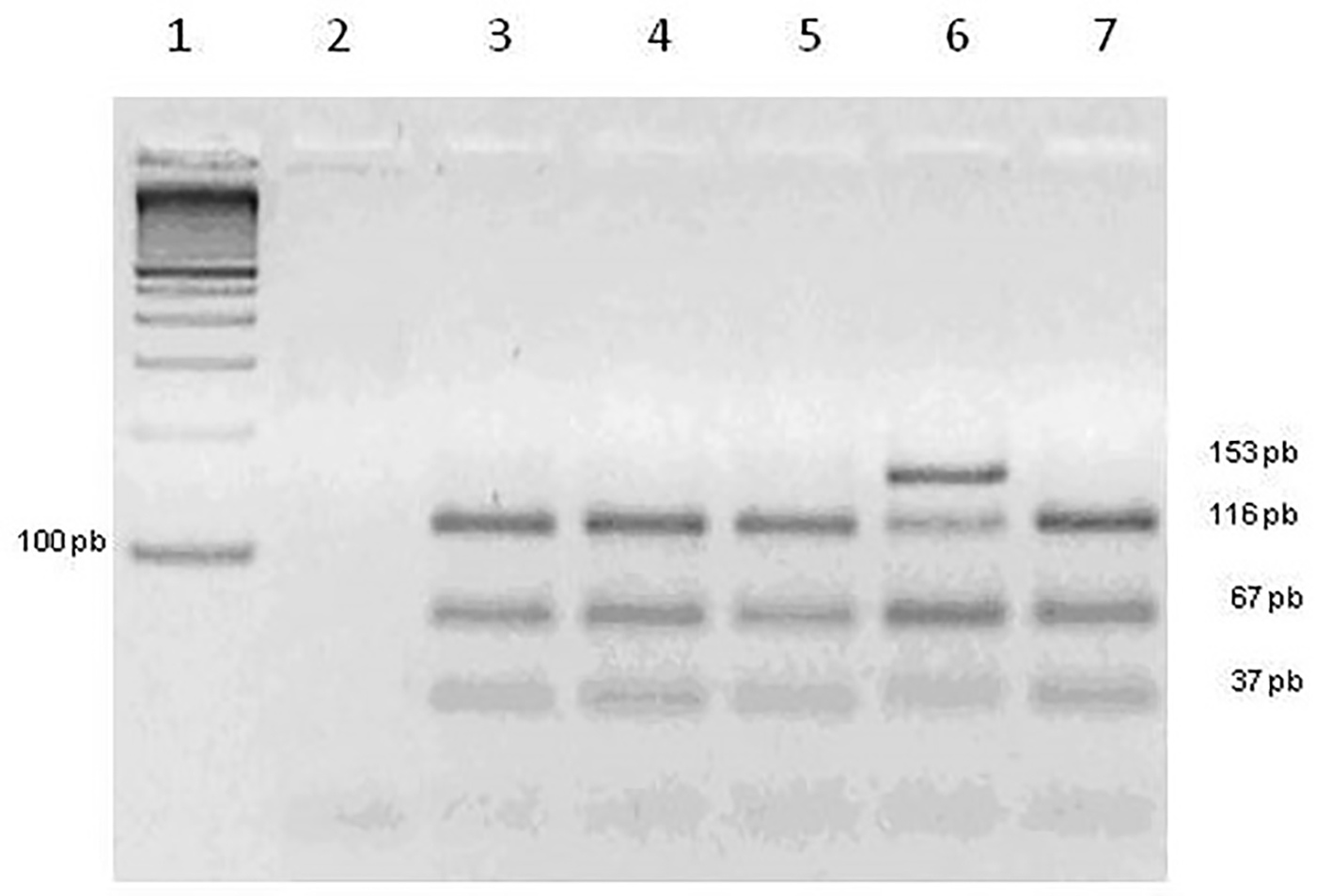 Click for large image | Figure 1. Agarose gel of FVL. Slot 1: molecular marker 100 pb. Slot 2: negative control. Slots 3, 4, 5 and 7: genotype homozygous wild. Slot 6: genotype heterozygous. |
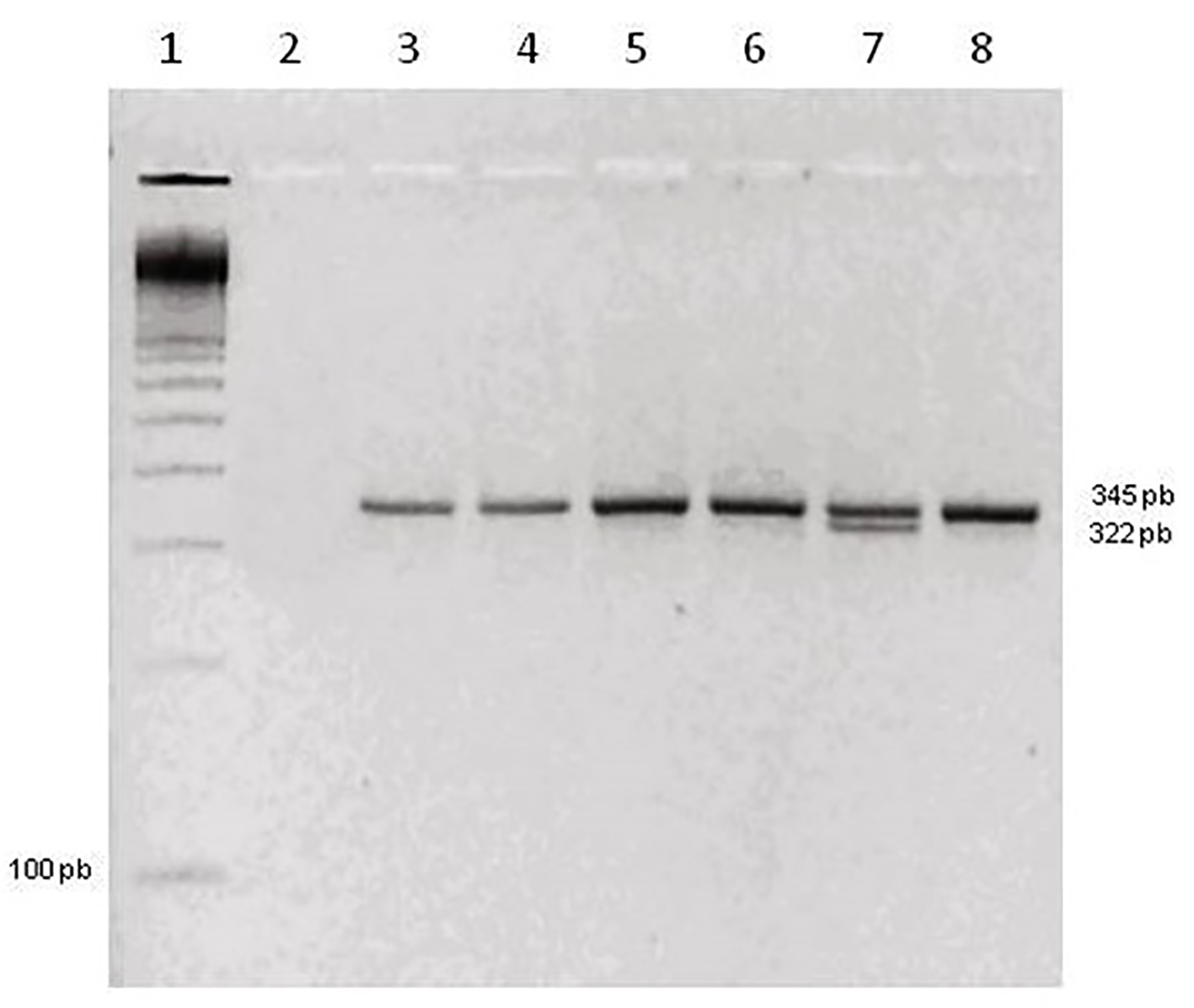 Click for large image | Figure 2. Agarose gel polymorphism in the prothrombin gene. Slot 1: molecular marker 100 pb. Slot 2: negative control. Slots 3, 4, 5, 6 and 8: genotype homozygous wild. Slot 7: genotype heterozygous. |
 Click to view | Table 2. Frequency of Polymorphisms in Abnormal and Normal Abortions |
| Discussion | ▴Top |
Several factors may be associated to the causes of abortions; however, no study has the chromosomal alterations of abortions before the study of polymorphisms, which is of great relevance, because more than 50% of chromosomal abnormalities lead to abortions. The cytogenetic study is considered as a prerequisite exam for investigating the causes of abortion, suggested by Moraes et al (2005) [5]. These authors demonstrated the fundamental importance of cytogenetic studies of chromosomal abnormalities in miscarriages samples, prior to investigating other possible causes in couple with a history of RSA. Some studies showed the cytogenetic abnormalities and hereditary thrombophilias in RSAs, the association with risk factors, between the presence of FVL, FII G20210A mutations and patients with normal karyotype [17-19]. In 1999, Meinardi et al [20] examined the FVL in mothers with spontaneous abortions and suggested the study of hereditary thrombophilia in fetus and of the clinical implications related with embryo genotype. Mahjoub et al (2005) [21] studied the association between genotypes and the FVL G1691A and prothrombin G20210A seen in women with history of recurrent idiopathic miscarriages. Because of no association between the presence of polymorphisms with recurrent pregnancy loss and maternal thrombotic events, in this study, screening of existence of APC resistance was recommended in women with recurrent miscarriages. Recently, Lino et al (2015) [17] showed no association between studied polymorphisms (FVL, PTM, MTHFR, C677T, MTHFR A1298C, and FXIII V34L) and the occurrence of recurrent abortion, but these results have a substantial value to carry out a meta-analysis. However none of these researches has tried to correlate with cytogenetic results of abortions samples. We performed comparisons between pre-determined groups, thus verifying whether these polymorphisms were present more in some than others. Regarding the FVL, this proved to be close to the significance levels in two of the eight analyzes. Comparing AA with NA, the results reached close to the significance level (P = 0.08) and the presence of FVL (percentage of heterozygotes) was higher in patients without cytogenetic alteration. The same was found in the comparison between the AA versus NHA groups (P = 0.048). Although a close difference to levels of significance in the comparison was not found between groups, NHA and AHA, NIA and AIA, NIA and AA, the percentage of heterozygous FVL was also higher in groups with the normal cytogenetic results. These results lead us to believe that the FVL can cause a predisposition to abortion, and the samples showed cytogenetic abnormalities, i.e., have no defined cause. In groups of AH versus AI and NHA and NIA was found more in FVL AH, but this difference did not have significance levels. These data suggest that this polymorphism may be more important in recurrent miscarriages. In the analysis between groups AHA EIA versus the frequency of FVL, it was close to 2.7% and 2.1%, respectively. The lower incidence of this polymorphism in cytogenetically abnormal miscarriages shows that it is probably not contributing to abortion in these cases and the loss was actually caused by chromosomal abnormalities. On the other hand, we can think that the presence of FVL polymorphism may cause a predisposition to abortion in patients without cytogenetic alterations, i.e. without giving a definite cause. The polymorphism in the prothrombin gene did not find statistically significant differences in any of the eight analyzes. However, the comparison between the AA and NA groups, NHA and AHA, AA and AA, and NIA and NHA, the frequency of this polymorphism was higher in normal karyotype groups. The polymorphism of prothrombin is a low frequency polymorphism in the general population ranging between 1% and 3% [22-24] and probably the correlation with abortion is only possible in work involving a larger sample. The NA was four times more likely to have at least one of the polymorphisms changed compared to the cases of AA (P = 0.014). The NHA was six times more likely to have one of the polymorphisms than AHA (P = 0.018). Similarly, comparison of AA with NHA (P = 0.01) showed the last five times more cases of polymorphic changes. Therefore, these data helped confirm that the molecular changes that lead to a hypercoagulable state are more common in normal karyotype abortions and are likely contributing to the genesis of abortion. The way to analyze the frequency data of multiple polymorphisms related to concurrently thrombophilia has already been performed by Coulam et al (2006) [25], who studied 10 polymorphisms and found no association when evaluated in isolation. Reports in the literature relevant to the study of polymorphisms in abortion material are scarce, as well as comparisons between groups with altered and the normal cytogenetics. In general, the studies compare the frequency of these polymorphisms in women with miscarriage and women with successful pregnancy [3, 26, 27]. Many cytogenetic abnormalities cause such cellular alterations that it explains the pregnancy loss. Once comparing findings in patients with and without cytogenetic alterations, we evaluated the role of polymorphisms in abortions: they are, normal or isolated. As noted, samples without chromosomal disorders have higher levels of these polymorphisms involved in coagulation phenomena, but in some groups, they were not sufficient to demonstrate significance, so these results merit confirmation in studies involving a larger number of cases. Fetal loss is a common event in medicine, but a lot of insecurity, depression and even feelings of guilt, a couple always shares, especially women, whose recurrent dream was interrupted. Therefore, research in this area, should be initiated by should be initiated by studying the possible causes of RSA, should be initiated with fetal and placental samples for karyotype analysis and polymorphisms and if necessary further studies of molecular biology to contribute to genetic counseling of the couple, are needed and also preparing them for a new attempt at successful pregnancy.
Conclusion
The FVL showed probable importance in the genesis of abortions due to its greater frequency of normal karyotype miscarriage. Once presence of both polymorphisms was significantly higher in normal karyotypes miscarriage than in abnormal, we can infer that the alterations are associated with the pathophysiology of abortions.
Acknowledgments
This study was supported by Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil.
Competing Interests
The authors declare that they have no competing interests.
Grant Support
None.
| References | ▴Top |
- Kupferminc MJ, Rimon E, Ascher-Landsberg J, Lessing JB, Many A. Perinatal outcome in women with severe pregnancy complications and multiple thrombophilias. J Perinat Med. 2004;32(3):225-227.
doi pubmed - Branch DW, Dudley DJ, Scott JR, Silver RM. Antiphospholipid antibodies and fetal loss. N Engl J Med. 1992;326(14):952; author reply 953-954.
pubmed - Mtiraoui N, Borgi L, Hizem S, Nsiri B, Finan RR, Gris JC, Almawi WY, et al. Prevalence of antiphospholipid antibodies, factor V G1691A (Leiden) and prothrombin G20210A mutations in early and late recurrent pregnancy loss. Eur J Obstet Gynecol Reprod Biol. 2005;119(2):164-170.
doi pubmed - Kalousek DK. Anatomic and chromosome anomalies in specimens of early spontaneous abortion: seven-year experience. Birth Defects Orig Artic Ser. 1987;23(1):153-168.
pubmed - Moraes AC, Moron AF, Hashimoto E, Silva IDG, Torloni MR, Souza MM, Patricio FRS. Cytogenetic and molecular evaluation of spontaneous abortion sample. Revista Brasileira de Ginecologia e Obstetricia. 2005;27:554-560.
- Mierla D, Szmal C, Neagos D, Cretu R, Stoian V, Jardan D. Association of Prothrombin (A20210G) and Factor V Leiden (A506G) with Recurrent Pregnancy Loss. Maedica (Buchar). 2012;7(3):222-226.
- Boyd TK, Redline RW. Chronic histiocytic intervillositis: a placental lesion associated with recurrent reproductive loss. Hum Pathol. 2000;31(11):1389-1396.
doi - Li TC, Spuijbroek MD, Tuckerman E, Anstie B, Loxley M, Laird S. Endocrinological and endometrial factors in recurrent miscarriage. BJOG. 2000;107(12):1471-1479.
doi - Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood. 1995;85(6):1504-1508.
pubmed - Girling J, de Swiet M. Inherited thrombophilia and pregnancy. Curr Opin Obstet Gynecol. 1998;10(2):135-144.
doi pubmed - Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3'-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88(10):3698-3703.
pubmed - Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368(9535):601-611.
doi - Simoni G, Brambati B, Danesino C, Rossella F, Terzoli GL, Ferrari M, Fraccaro M. Efficient direct chromosome analyses and enzyme determinations from chorionic villi samples in the first trimester of pregnancy. Hum Genet. 1983;63(4):349-357.
doi pubmed - Simoni G, Terzoli G, Rossella F. Direct chromosome preparation and culture using chorionic villi: an evaluation of the two techniques. Am J Med Genet. 1990;35(2):181-183.
doi pubmed - Eiben B, Goebel R, Hansen S, Hammans W. Early amniocentesis - a cytogenetic evaluation of over 1500 cases. Prenat Diagn. 1994;14(6):497-501.
doi pubmed - Shaffer L, Tommerup N: ISCN (2005): International System of Human Cytogenetic Nomenclature. 1 edition edn; 2005.
- Lino FL, Traina E, Barreto JA, Moron AF, Mattar R. Thrombophilic mutations and polymorphisms, alone or in combination, and recurrent spontaneous abortion. Clin Appl Thromb Hemost. 2015;21(4):365-372.
doi pubmed - Carp H, Salomon O, Seidman D, Dardik R, Rosenberg N, Inbal A. Prevalence of genetic markers for thrombophilia in recurrent pregnancy loss. Hum Reprod. 2002;17(6):1633-1637.
doi pubmed - Elshafaay AE, Mostafa FM, Al-Awadi SA, Akbar LB, Abel Wahab MM, Abd El-Aziz HM, Naguib KK. Cytogenetic Abnormalities and Herdetiry Thrombophilias among the Abortees. J Egypt Public Health Assoc. 2008;83(5-6):389-402.
pubmed - Meinardi JR, Middeldorp S, de Kam PJ, Koopman MM, van Pampus EC, Hamulyak K, Prins MH, et al. Increased risk for fetal loss in carriers of the factor V Leiden mutation. Ann Intern Med. 1999;130(9):736-739.
doi pubmed - Mahjoub T, Mtiraoui N, Tamim H, Hizem S, Finan RR, Nsiri B, Almawi WY. Association between adverse pregnancy outcomes and maternal factor V G1691A (Leiden) and prothrombin G20210A genotypes in women with a history of recurrent idiopathic miscarriages. Am J Hematol. 2005;80(1):12-19.
doi pubmed - Rosendaal FR, Doggen CJ, Zivelin A, Arruda VR, Aiach M, Siscovick DS, Hillarp A, et al. Geographic distribution of the 20210 G to A prothrombin variant. Thromb Haemost. 1998;79(4):706-708.
pubmed - Brenner B, Sarig G, Weiner Z, Younis J, Blumenfeld Z, Lanir N. Thrombophilic polymorphisms are common in women with fetal loss without apparent cause. Thromb Haemost. 1999;82(1):6-9.
pubmed - Finan RR, Tamim H, Ameen G, Sharida HE, Rashid M, Almawi WY. Prevalence of factor V G1691A (factor V-Leiden) and prothrombin G20210A gene mutations in a recurrent miscarriage population. Am J Hematol. 2002;71(4):300-305.
doi pubmed - Coulam CB, Jeyendran RS, Fishel LA, Roussev R. Multiple thrombophilic gene mutations rather than specific gene mutations are risk factors for recurrent miscarriage. Am J Reprod Immunol. 2006;55(5):360-368.
doi pubmed - Sullivan AE, Nelson L, Rice JA, Porter TF, Branch DW, Silver RM. The factor V Leiden and the G20210A prothrombin gene mutations are rare in women with fetal death. Am J Reprod Immunol. 2005;54(1):1-4.
doi pubmed - Sottilotta G, Oriana V, Latella C, Luise F, Piromalli A, Ramirez F, Mammi C, et al. Genetic prothrombotic risk factors in women with unexplained pregnancy loss. Thromb Res. 2006;117(6):681-684.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.







