| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 1, Number 2-3, June 2012, pages 40-45
Maternal Inflammatory Response in Severe Preeclamptic and Preeclamptic Pregnancies
Merih Bayrama, Mehmet Suhha Bostancib, e, M. Baran Celtemena, Emin Umit Bagrlaclkc, Melek Yamanc, Fusun Civild
aGazi University, Faculty of Medicine Department of Obstetrics and Gynecology, Ankara, Turkey
bSakarya Education and Research Hospital, Sakarya, Turkey
cGazi University, Faculty of Medicine Department of Immunology, Ankara, Turkey
dGazi University, Faculty of Medicine Department of Public Health, Ankara, Turkey
eCorresponding author: Mehmet Suhha Bostanci, Sakarya Egitim Arastlrma Hastanesi Kadln Hastallklarl ve Dogum Klinigi Korucuk Kampusu. Sakarya, Turkey
Manuscript accepted for publication June 25, 2012
Short title: Maternal Inflammatory Response
doi: https://doi.org/10.4021/jcgo44w
| Abstract | ▴Top |
Background: The aim of this study was to use serum levels of IL-6, IL-8, IL-1β, TNF-α, CD40L and HSCRP as an in vitro inflammatory stimulus in order to profile the inflammatory response of whole blood from pregnant women with preeclampsia and severe preeclampsia, and to determine whether this functional response fits with imbalance.
Methods: The preeclampsia group (n = 27) and severe preeclampsia group (n = 21) were generated by matching the diagnostic criteria of the International Society for the Study of Hypertension in Pregnancy (ISSHP). Control group (n = 21) was matched for gestational stage, maternal age, and parity. The panel of cytokines analyzed included interleukin IL-1β, IL-6, IL-8, and TNF-α, HSCRP, CD40L for all groups.
Results: There were statistically high values of IL-6 (P < 0.003) and TNF-α (P < 0.003) at preeclampsia group versus control group, the significantly high values of IL-6 (P < 0.001) and IL-1β (P < 0.005) was found at the severe preeclampsia group versus control group. There were no significant difference between the groups of preeclampsia and severe preeclampsia for IL-6, IL-8, IL-1β, TNF-α, CD40L and HSCRP.
Conclusion: Increasing gestational age in normal pregnancy features an increased inflammatory responsiveness, which is potentially a preparatory mechanism for maternal sensitivity to the fetal triggers of labour. This increase in response is mirrored by women with preeclampsia, highlighting the importance of stratifying patients according to the timing the onset of disease given their inherent differences in inflammatory function. It is important to know determinative factors for changing preeclampsia to severe preeclampsia. Increased level of IL-6 and TNF-α may play on the role of triggering factors for preeclampsia and severe preeclampsia. So it must be studied to be determining cut off value of these parameters.
Keywords: Preeclampsia; Severe preeclampsia; IL-6; IL-1β; IL-8; TNF-α; HSCRP; CD40L
| Introduction | ▴Top |
Preeclampsia (PE) is pregnancy related disorder characterized by hypertension and proteinuria that occurs after 20 weeks of gestation [1, 2]. PE is the third most common cause of maternal death and accounts for up to 40.000 pregnancy related death per year in the developing countries [2]. Severe PE is also associated with both maternal and fetal risks, with serious maternal complications in 27% of cases and overall 34% risk for neonatal complications [3].
Despite intensive research efforts, the etiology and pathogenesis of preeclampsia remain unclear. It has multifactorial etiology involving both genetic and environmental factors, and is associated with underlying inflammatory dysfunction. Increasing evidence suggests that an excessive maternal systemic inflammatory response to pregnancy with activation of both the innate and adaptive arms of the immune system is involved in the pathogenesis of the disease [4, 5]. Pregnancy itself has been described as a state of controlled mil inflammation situation [6]. During pregnancy a generalized maternal inflammatory response has been shown to occur during preeclampsia together with increased levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 [7, 8]. It has been hypothesized that the exaggerated systemic inflammation seen in preeclampsia may result from a decompensation of one or more of the maternal protective systems [5]. This situation is suggesting that PE is associated with a reversal in T-helper (Th) 1: Th2 balance, thereby featuring Th1-type predominance. This activated Th1-type response is supported by further studies reporting elevated levels of pro-inflammatory cytokines (e.g., TNF-α) or their inducers (e.g., IL-12) in association with PE [5, 9, 10].
The aim of this study was therefore to demonstrate difference blood levels of IL-6, IL-8, IL-1β, TNF-α, CD40 ligand (CD40L), Highly sensitive C-reactive protein (HSCRP) for pregnant women with preeclampsia and severe preeclampsia, and to determine whether this functional response fits with imbalance.
| Materials and Methods | ▴Top |
Subjects
Twenty seven preeclamptic patients, 21 severe preeclamptic patients and 21 healthy pregnant women with uncomplicated pregnancies were involved in the study. The study participants were enrolled in the Department of Obstetrics and Gynecology of the Gazi University Hospital, at the Gazi University, Ankara, Turkey. Ethical approval for the study was obtained from the Gazi University Medical Faculty Regional Ethics Committee and all participants gave written informed consent.
The preeclampsia group comprised of women (n = 27) matching the diagnostic criteria of the International Society for the Study of Hypertension in Pregnancy (ISSHP): two blood pressure readings of ≥ 140/90 mmHg took at least with > 300 mg proteinuria over 24 hours or +1 on dipstick analysis [11]. The severe preeclampsia group comprised of women (n = 21) matching the diagnostic criteria of the ISSHP: two blood pressure readings of ≥ 160/110 mmHg took at least with > 5 g proteinuria over 24 hours or +3 on dipstick analysis [11]. Control group patients (n = 21) were matched for gestational stage, maternal age, and parity. The exclusion criteria for this group included concurrent medical problems that may result in disordered inflammatory response, such as diabetes, autoimmune disease and spontaneous rupture of membranes.
Biological samples
Ten mL whole blood was collected from the antecubital vein of subjects in lithium heparin tubes. These were centrifuged at 2500 rpm for 15 min in order to isolate the supernatant, which was frozen at -86 °C until analyzed for cytokine profiles.
Cytokine analysis
Enzyme immunoassay (EIA)
In the present study, the panel of cytokines analyzed included IL-1β, IL-6, IL-8, TNF-α, HSCRP, CD40L. Cytokine release was tested by using ultrasensitive enzyme-linked immunosorbent assay (ELISA) kits according to the Manufacturers’ recommendations (Invitrogen. Carlsbad, CA, USA). Briefly, 100 micro liter human serum per sample was mixed with sample buffer in individual wells of microtiter plates provided by the manufacturer as part of the kit content. Two wells/samples were used. The mixture was incubated for 2 hours at room temperature. Plates were washed 4 times using a Tecan, Columbus plate washer (Tecan, Mannedorf, Switzerland). Wells were treated with a biotin-conjugated secondary antibody by incubating for two hours at room temperature. Plates were washed for 4 times and incubated with streptavidin-HRP. Color development was observed in the presence of substrate and measured at 450 nm by using a Biotek, Synergy HT multi-mode microplate reader (Biotek, Winooski, VA, USA).
Statistical analysis
To compare continuous variables between groups, the Mann-Whitney U test was applied, whereas to compare them between multiple groups, the Kruskal-Wallis analysis of variance of ranks test was performed. Contingency tables, Chi-square, and Wilcoxon W test were employed for comparisons of proportions. Analysis was conducted with SPSS V.11.5 (SPSS, Chicago, IL). For all statistical analyses, P < 0.05 was considered statistically significant.
| Results | ▴Top |
The demographics and the clinical features of the groups have been outlined in Table 1. There was no significant difference in maternal age, gestational age, parity. Women with preeclampsia showed significantly higher urinary concentrations of protein compared with women with normal pregnancies (P < 0.005). In severe preeclamptic cases, this situation was even more significant in the comparison groups of normal and preeclamptic patients.
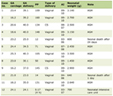 Click to view | Table 1. Demographic Properties of Groups |
For all the factors tested, i.e., IL-6, IL-8, IL-1β, TNF-α, CD40L and HSCRP, there was no significant difference between the groups of preeclampsia and severe preeclampsia (Table 2). While there was statistically high values of IL-6 (P < 0.003) and TNF-α (P < 0.003) at preeclampsia group versus control group, the significantly higher values of IL-6 (P < 0.001) and IL-1β (P < 0.005) was found in the severe preeclampsia group versus control group.
 Click to view | Table 2. Factors Measurements in Preeclampsia and Severe Preeclampsia |
| Discussion | ▴Top |
The precise etiology of preeclampsia is still unknown, one key factor of the involved mechanisms is exaggerated systemic inflammation resulting from decomposition of one or more of the maternal protective systems [5]. During pregnancy is known to increase in response to inflammation. A progressive monocytic activation in the circulation of pregnant women compared to non-pregnant women was shown [12]. Monocytes from preeclamptic patients demonstrated a further increase in basal intracellular reactive oxygen species, a higher synthesis of interleukin IL-1β, IL-6 and IL-8 compared to monocytes from healthy pregnant women [5, 8, 9]). There is a balance for the production and release of immunoregulatory cytokines by a variety of immune effector cells, which operate in synergistic/inhibitory interaction-based networks according to the pro- (TNF-α, IL-1, IL-6, interferon (IFN-γ) (Th1-type) or anti-inflammatory (IL-4, IL-13) (Th2-type) nature of the cytokines involved [13]. In a normal pregnancy a Th2-type (anti-inflammatory) response is predominant compared to a Th1-type (pro inflammatory) response [14]. In cases of preeclampsia, there is an alteration in the balance between Th1 and Th2-type responses. By contrast, patients with preeclampsia have been shown to have higher levels of serum pro-inflammatory [15, 16] and lower levels of anti-inflammatory cytokines [17] compared with healthy pregnant, thereby featuring a Th1-type predominance. Elevated levels of pro-inflammatory cytokines (TNF-α) or their inducers (IL-12) are also supported this activated Th1-type response [9, 10, 18].
In this study, IL-6 was found high both preeclampsia and severe eclampsia when compared to the normal pregnancies (P < 0.003, P < 0.001). The finding for IL-6 is in line with previous reports on elevated plasma levels of IL-6 in women with preeclampsia compared with normal pregnancies [18-20]. In contrast, Al-Othman et al found no difference in IL-6 levels in serum between preeclampsia and normal pregnancy [21]. We found increased levels of TNF-α for women with preeclampsia compared with normal pregnancies (P < 0.003). As evaluation of IL-6 levels as a result of a lack of difference between severe preeclampsia and normal pregnancy was remarkable. These results were convenient with the literature. Serum levels of TNF-α being typically are elevated in preeclamptic patients in association with the disorder. As a result of the inadequate trophoblast invasion and placental hypoxia, TNF-α secretion is increased in placental tissue following hypoxia-reoxygenation in vitro for preeclamptic patient [22]. Trophoblast cells trigger peripheral monocyte inflammatory responsiveness. As such, they can increase cytokine production (including IL-6, IL-8 and TNF-α). But these effects may be very aggressive in severe preeclampsia, and the triggered value is determinative value for it, this may be cut off value. IL-1β is a known inducer of monocyte chemotactic protein (MCP) 1 expression [23] and its role in pathogenesis of preeclampsia was shown previously [15, 24]. Our data was determined significantly higher blood levels of IL-1β in severe preeclampsia (P < 0.005) when compared to normal pregnant women, so this may be convinced of seriousness. In in vitro models of atherosclerosis, MCP-1 include oxidative stress operates through endothelial nuclear transcription factor-1B activity [25].
The near-term to delivery, increased maternal inflammatory response is known [6]. Physiological increase in maternal inflammatory responsiveness is unclear, although it is likely to have a role in preparation for labour. Increases in pro-inflammatory cytokines (e.g. TNF-α, IL-1β) are also required for similar processes, such as by stimulating production of matrix metalloproteinase (MMP)-1, MMP-3, MMP-9 which need for cervical ripening [26, 27]. In support of preeclampsia involving an exacerbation of the physiological increase of this inflammatory responsiveness, which may be due to the comparatively small numbers of patients involved.
C-reactive protein (CRP) is known marker of systemic inflammation and which was found be higher in pregnant than in nonpregnant women [28]. HSCRP levels have been better sensitivity in establishing inflammation than levels of CRP [29]. Serum levels of HSCRP had been positively correlated with pregnancy duration in normal, and had been found higher in preeclamptic pregnants to the normal pregnants [29], and they suggested that it could be predicted by evaluating HSCRP in severe preeclampsia. But in this study, it was not any important levels of HSCRP at all groups.
The ligand for CD40 is a glycoprotein structurally related TNF-α which is expressed on T cells, mast cells, and natural killer cells [30]. The immune modulator pair CD40 and CD40L has been proposed to be an important link between inflammation and thrombosis [31]. Henn et al found high levels of soluble CD40L failed to induce an inflammatory reaction [32]. The possible role of soluble CD40L as a proinflammatory or a limiting factor of inflammation. The higher levels of soluble CD40L in pregnant with preeclampsia compared with those in normotensive pregnants could be indicated an exaggerated activation of plateletes and endothelial cells in preeclampsia [33]. But we didn’t find in this levels statistically meaningful between all groups. We were persuaded that there will be useful to studies with much more subjects in it.
In conclusion, increasing gestational age in normal pregnancy features an increased inflammatory responsiveness, which is potentially a preparatory mechanism for maternal sensitivity to the fetal triggers of labour. This increase in response is mirrored by women with preeclampsia, highlighting the importance of stratifying patients according to the timing the onset of disease given their inherent differences in inflammatory function. It is important to know determinative factors for changing preeclampsia to severe preeclampsia. We are suspicious about the increasing the value of IL-6 and TNF-α on the role of triggering factors for preeclampsia and severe preeclampsia. So it must be studied to be determine cut off value of these parameters.
| References | ▴Top |
- Neilson JP. Pre-eclampsia and Eclampsia. In: Why Mothers Die Confidential Enquiry into Maternal and Child Health 2002-2002. London, UK: RCOG Press; 2004. pp. 7985.
- ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99(1):159-167.
pubmed - Hall DR, Odendaal HJ, Kirsten GF, Smith J, Grove D. Expectant management of early onset, severe pre-eclampsia: perinatal outcome. BJOG. 2000;107(10):1258-1264.
pubmed doi - Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med. 2007;28(2):192-209.
pubmed doi - Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2 Pt 1):499-506.
pubmed doi - Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today. 1999;20(3):114-118.
pubmed doi - Luppi P, Deloia JA. Monocytes of preeclamptic women spontaneously synthesize pro-inflammatory cytokines. Clin Immunol. 2006;118(2-3):268-275.
pubmed doi - Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response—a review. Placenta. 2003;24 Suppl A:S21-27.
pubmed - Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1994;170(6):1752-1757; discussion 1757-1759.
pubmed - Azizieh F, Raghupathy R, Makhseed M. Maternal cytokine production patterns in women with pre-eclampsia. Am J Reprod Immunol. 2005;54(1):30-37.
pubmed doi - Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20(1):IX-XIV.
pubmed - Shibuya T, Izuchi K, Kuroiwa A, Okabe N, Shirakawa K. Study on nonspecific immunity in pregnant women: increased chemiluminescence response of peripheral blood phagocytes. Am J Reprod Immunol Microbiol. 1987;15(1):19-23.
pubmed - Oppenheim JJ, Feldman M. An introduction to the role of cytokines in innate host defence and adaptive immunity. In: Oppenhiem JJ, Feldman M, Durum SK, Hirano T, Vilcek J, Nicola NA, eds. A Compendium of Cytokines and other Mediators of Host Defense, Volume 1 (First Edition) San Diego: San Diego Academic Press; 2000:3-20.
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353-356.
pubmed doi - Greer IA, Lyall F, Perera T, Boswell F, Macara LM. Increased concentrations of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism for endothelial dysfunction? Obstet Gynecol. 1994;84(6):937-940.
pubmed - Kocyigit Y, Atamer Y, Atamer A, Tuzcu A, Akkus Z. Changes in serum levels of leptin, cytokines and lipoprotein in pre-eclamptic and normotensive pregnant women. Gynecol Endocrinol. 2004;19(5):267-273.
pubmed doi - Rein DT, Breidenbach M, Honscheid B, Friebe-Hoffmann U, Engel H, Gohring UJ, Uekermann L, et al. Preeclamptic women are deficient of interleukin-10 as assessed by cytokine release of trophoblast cells in vitro. Cytokine. 2003;23(4-5):119-125.
pubmed doi - Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40(2):102-111.
pubmed doi - Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102(1):20-25.
pubmed doi - Madazli R, Aydin S, Uludag S, Vildan O, Tolun N. Maternal plasma levels of cytokines in normal and preeclamptic pregnancies and their relationship with diastolic blood pressure and fibronectin levels. Acta Obstet Gynecol Scand. 2003;82(9):797-802.
pubmed doi - Al-Othman S, Omu AE, Diejomaoh FM, Al-Yatama M, Al-Qattan F. Differential levels of interleukin 6 in maternal and cord sera and placenta in women with pre-eclampsia. Gynecol Obstet Invest. 2001;52(1):60-65.
pubmed doi - Hung TH, Charnock-Jones DS, Skepper JN, Burton GJ. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol. 2004;164(3):1049-1061.
pubmed doi - Lee SA, Fitzgerald SM, Huang SK, Li C, Chi DS, Milhorn DM, Krishnaswamy G. Molecular regulation of interleukin-13 and monocyte chemoattractant protein-1 expression in human mast cells by interleukin-1beta. Am J Respir Cell Mol Biol. 2004;31(3):283-291.
pubmed doi - Rinehart BK, Terrone DA, Lagoo-Deenadayalan S, Barber WH, Hale EA, Martin JN, Jr., Bennett WA. Expression of the placental cytokines tumor necrosis factor alpha, interleukin 1beta, and interleukin 10 is increased in preeclampsia. Am J Obstet Gynecol. 1999;181(4):915-920.
pubmed doi - Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest. 2001;107(3):255-264.
pubmed doi - Cockle JV, Gopichandran N, Walker JJ, Levene MI, Orsi NM. Matrix metalloproteinases and their tissue inhibitors in preterm perinatal complications. Reprod Sci. 2007;14(7):629-645.
pubmed doi - Watari M, Watari H, DiSanto ME, Chacko S, Shi GP, Strauss JF, 3rd. Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am J Pathol. 1999;154(6):1755-1762.
pubmed doi - Watts DH, Krohn MA, Wener MH, Eschenbach DA. C-reactive protein in normal pregnancy. Obstet Gynecol. 1991;77(2):176-180.
pubmed doi - Hwang HS, Kwon JY, Kim MA, Park YW, Kim YH. Maternal serum highly sensitive C-reactive protein in normal pregnancy and pre-eclampsia. Int J Gynaecol Obstet. 2007;98(2):105-109.
pubmed doi - Hollenbaugh D, Grosmaire LS, Kullas CD, Chalupny NJ, Braesch-Andersen S, Noelle RJ, Stamenkovic I, et al. The human T cell antigen gp39, a member of the TNF gene family, is a ligand for the CD40 receptor: expression of a soluble form of gp39 with B cell co-stimulatory activity. EMBO J. 1992;11(12):4313-4321.
pubmed - Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111-135.
pubmed - Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391(6667):591-594.
pubmed doi - Oron G, Ben-Haroush A, Hod M, Orvieto R, Bar J. Serum-soluble CD40 ligand in normal pregnancy and in preeclampsia. Obstet Gynecol. 2006;107(4):896-900.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.







