| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Review
Volume 3, Number 1, February 2014, pages 1-7
Current Concept of Bacterial Vaginosis in Cervical Cancer
Biswa Mohan Biswala, c, Kirnpal Kaur Banga Singhb, Mazian Binti Ismaila, Muhammad Irfan Bin Abdul Jalala, Engku Ibrahim Syubli Bin Engku Safruddinb
aDepartment of Nuclear medicine, Radiotherapy & Oncology, School of Medical Sciences, Health Campus, Universiti Sains Malaysia, Kelantan, Malaysia
bDepartment of Medical Microbiology & Parasitology, School of Medical Sciences, Health Campus, Universiti Sains Malaysia, Kelantan, Malaysia
cCorresponding author: Biswa Mohan Biswal, Department of Nuclear medicine, Radiotherapy & Oncology, School of Medical Sciences, Health campus, Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia
Manuscript accepted for publication August 2, 2013
Short title: Bacterial Vaginosis in Cervical Cancer
doi: https://doi.org/10.14740/jcgo175w
- Abstract
- Introduction
- Incidence and Prevalence of BV in Cervical Cancer
- Pathogenesis
- Relation to Host Immunity
- Difference Between Commensals and Invasive Bacterial Infection
- Implication to Treatment
- Conclusion
- References
| Abstract | ▴Top |
Invasive cervical cancer is often associated with bacterial vaginosis (BV) caused by both non-pathogenic and pathogenic bacteria and other microorganisms. BV goes un-noticed during the clinical course of cervical cancer. The incidence of BV is very high among women from developing countries with poor genital hygiene. Maintainence of normal vaginal ecosystem in healthy vagina is through control of optimal ratio between non-pathogenic and pathogenic organisms. Cervical cancer causes disruption of the normal vaginal and cervical mucosa leading to alteration of ratio between commensals and pathogenic organisms. The natural vaginal microflora is being dominated by lactobacilus species compared to pathogenic aerobic and anaerobic microorganisms. Disruption of vaginal microenvironment leads to change in vaginal flora and associated inflammation. There are very limited literature available on the exact incidence of BV in cervical cancer patients. Increased nitrosamine content in BV could possibly progress to higher DNA damage, change in cytokine profile thus compromise immune defence against human papilloma virus infection. Both radiotherapy and chemotherapy are potential immunosuppressive agent thus could facilitate spread of endogenous bacteria to manifest as poor outcome to treatment secondary to tissue hypoxia and hypoperfusion. This article is a review of current literature associated with BV in cervical cancer.
Keywords: Bacterial vaginosis; Cervical cancer; HPV infection; Treatment; Outcome
| Introduction | ▴Top |
Cervical cancer is the second common malignancy after breast cancer among women which accounts for more than half a million new cases and quarter of a million deaths annually, and also the second most common virus-related cancer among women in developing countries [1]. A report in 2004 indicated that there were approximately 12,000 cases of invasive cervical cancer with 3,850 deaths among American women [2]. However, prevalence of cervical cancer reported by World Health Organization (WHO) in developing countries remains incomplete and fragmentary. Infection of high-risk human papillomavirus is strongly associated with the development of invasive cervical cancer and its precursor lesions, cervical intraepithelial neoplasia (CIN) [3]. Infact, still, not all of HPV infection leads to cervical cancer, suggesting that other cofactors could be present in the development of malignancy. Thus, other known risk factors including young age at first intercourse, cigarette smoking, race, multiparity, infection with sexual transmitted disease agent such as Chlamydia trachomatis, herpes simplex virus (HSV) and bacterial vaginosis (BV) [4, 5] were recognized. BV is a common vaginal disorder among women resulted in reduction of naturally occurring lactobacilli growth (normal flora) and is often replaced with a mixed, predominantly anaerobic bacteria consisting of Gardnerella vaginalis, Mobiluncus spp., Atopobium vagina, Mycoplasma hominis, Bacteroides spp. and Prevotella spp. [6, 7]. This situation may occur when microenvironment of vagina is disturbed and lead to certain clinical presentation including foul smelling discharge, alkaline vaginal pH > 4.5, a positive amine “whiff” test and the presence of clue cells on a wet smear [8]. These are the so-called “Amsel criteria” [9]. Local growth harbor bacterial growth in the vaginal cavity, leading to pelvic inflammatory disease and hydrometra.
BV is known to be associated with some gynecologic and obstetric complications such as postoperative infections, preterm labor during delivery, pelvic inflammation disease (PID), cervicitis, chorioamnionitis and premature rupture of membranes [10]. As abnormal microflora can produce carcinogenic nitrosamines and stimulate the release of cytokines, such as interleukin-1b, it has been suggested that BV may be important in the development of cervical cancer [11]. Carcinogenic nitrosamines increase the probability of DNA damage and an altered cytokine profile may reduce immune’s system ability to eliminate HPV infection. Thus, the changes may create a conducive environment for cancer development [12]. The Centers for Disease Control and Prevention (CDC), which is a part of the principal agency in the United States government for protecting the health and safety of all Americans and for providing essential human services, have included BV on their list of emerging infectious diseases [13].
| Incidence and Prevalence of BV in Cervical Cancer | ▴Top |
So far, the association of BV and development of cervical cancer is still remain unclear and conflicting. Some studies show the association of bacterial vaginal infection with cervical cancer [14] and other studies show no association at all [15]. However, the possibility still exist that BV is in some way associated with the development of CIN, as a cofactor to human papillomaviruses (HPV). Therefore, BV was taken into consideration in series of studies on CIN [10, 16]. In previous studies, the prevalence of BV in patients varies; 32-64% from STD clinics, 12-25% from gynecology outpatients clinics, 10-26% from antenatal clinics [17-20] in their reports indicate the similarity of BV prevalence in both, 18% of women with squamous intraepithelial lesion (SIL) and 12% of women without SIL. However, a higher rate of BV (33%) was found among women with high-grade SIL [20]. Besides, study in Japan by Mikamo et al [21] indicates that 50% of Gardnerella vaginalis and 80% of BV including other co-existing bacterial species was detected in cervical cancer patients. The different prevalence of BV among these studies might be due to that different technique, diagnostic criteria and clinician’s opinions in each study (Table 1) [15, 22-32]. For example, the prevalence obtained using Nugent’s criteria was consistently higher as opposed to studies using clinical “Amsel criteria” [7].
 Click to view | Table 1. Characteristics of Selected Studies in BV |
| Pathogenesis | ▴Top |
The pathogenesis of BV in cervical cancer is complex. BV is not attributable to a single causative organism but it’s due to immense overgrowth of composite of flora including G. vaginalis, Peptostreptococci, Bacterioides spp., Mobiluncus spp., Mycoplasma and Ureaplasma urealyticum. Fusobacterium and Atopobium vagina are also commonly found in BV. Inflammation plays a little role since this disease is due to the disruption of the vaginal microenvironment rather than a true tissue’s infective state. This overgrowth shifts the balance from Lactobacillus-predominated vaginal ecosystem to microenvironment with anaerobic bacteria dominance. This conclusion is corroborated by experimental studies conducted in humans and animal models that demonstrated upon vaginal inoculation with a single vaginosis-causing bacterial species, BV will rarely occur.
The factors that lead to anaerobes bacterial overgrowth remain uncertain. Increased availability of the substrates, raised in pH and loss of Lactobacillus spp. were implicated for BV because the vagina of normal women were predominantly inhabited by Lactobacillus spp. that produced hydrogen peroxide which play an important role in suppressing the overgrowth of anaerobes either directly or by producing a hydrogen-halide complex catalyzed by naturally-occuring cervical peroxidase. Following the anaerobic bacteria overgrowth, there will be an elevated polyamine production by the anaerobes, enhanced by the decarboxylases which broke down the vaginal peptides into polyamines. These polyamines along with vaginal organic acids (acetic and succinic acids) are cytotoxic to the vaginal cells, leading to the vaginal cell’s exfoliation manifesting as vaginal discharge which is malodorous and volatile due to its high amine content.
It has been hypothesized that BV results in cervical cancer through an increase in vaginal nitrosamines and altered cytokine profiles [12]. The presence of raise nitrosamines in vagina will lead to higher probability of DNA damage and change in cytokine profiles will cause altered response of immune system to clear up HPV infection. Therefore, BV will interact with HPV infection with the consequence of higher risk of developing cervical cancer among those with BV and HPV co-infection than those with a mono-infection. However, other infections for instance Candida was not associated with the development of CIN or cervical carcinoma [33].
Nevertheless, the causal relationship between BV-HPV interaction and cervical cancer development may not be entirely infallible since the causation may be bidirectional due to reverse causality. Owing to the relative immunodeficiency state in advanced cancer stage, BV thrives and therefore seems to be antecedent factor of cervical carcinoma whilst in fact the opposite is true. It’s equally coherent to postulate that BV-HPV infection is a bona-fide cause of cervical carcinoma. Unless there’s a properly executed and methodologically sound cohort study, the temporal order between BV-HPV and cervical carcinoma cannot be fully ascertained.
Besides that, BV is diagnosed by a multitude of diagnostic criteria. The most common ones used are Amsel and Nugent diagnostic criteria (Table 2) [34]. However, several studies for example two studies conducted in Netherlands, have used a unique diagnostic system called KOPAC to diagnose BV which was subsequently termed as vaginal dysbacteriosis, a completely different concept since its diagnosis is based on microscopic appearance rather than clinical Amsel or Nugent criteria. Therefore, the heterogeneity of diagnostic criteria further hampered the unifying conclusion on the causative potential of BV as cervical carcinoma’s etiology.
 Click to view | Table 2. Amsel and Nugent Diagnostic Criteria |
| Relation to Host Immunity | ▴Top |
Patients with CIN and BV exhibited raised level of interleukin (IL) 1, IL-6, IL-8, IL-10 and nitric oxide (NO) in both endocervical (EC) and vaginal secretions. Apart from that, bacteria in BV made negligible amount of lactic acid, while producing massive amount of immunomodulatory substances such as proteases, sialydases, succinate and possesses other inflammatory-inducing components for instance lipoteichoic acid (LA), peptidoglycans (PG) and lipopolysaccharides (LPS). These proinflammatory cytokines produced in response to the above substances and components promote the growth of neoplastic cells both in vitro and in vivo. Besides that, IL-6 and IL-8 have proangiogenic properties which encouraged the tumorigenesis of solid tumor such as non-small cell carcinoma of the lung and glioblastoma. Therefore, the elevated level of IL-6 and IL-8 may play a role in tumor angiogenesis and driver of neoplastic growth. Nevertheless, the inflammatory events might also be incited by other factors such as personal hygiene and sexual activities, and therefore these two factors were also cofounders for cervical carcinoma. Therefore, the purpose of establishing BV as the main driver for the activation of inflammatory cascades with ensuant development of cervical carcinoma remains elusive.
The role of infectious agents, particularly BV in upregulating integrin expression should also be elucidated. It has been recognized that integrin expression was regulated by cytokines such as transforming growth factor beta (TGF-beta). The signaling pathway of TGF-beta was hypothesized to be activated by various microbial components acting through Toll-like Receptor (TLR). Upon activation, TGF-beta will stimulate the expression of integrin which plays a major role in cervical carcinoma pathogenesis by recruiting and phosphorylating focal adhesion kinase (FAK), which is in turn activating Src and c-Jun N terminal kinase (JNK) together with P-I3K phosphorylation. This will culminate in the activation of protein Kinase B/AKB, Rac and extracellular signal regulated kinase (ERK). Since these events regulate the cell cycle, upregulation of beta 6 integrin will promote tumorigenesis through perpetuating the proliferation and survival of cancer cells.
| Difference Between Commensals and Invasive Bacterial Infection | ▴Top |
In female genital tract the differences between commensals and pathogens which may lead to invasive bacterial infection is complex. The essence of the argument lies in the fact that pathogens are not only defined by the microorganism’s type and its intrinsic virulence but also by the intricacies of the microfloral ecosystem in the vagina which is dependent on the relative dominance of bacterial species in the vaginal tract. According to the conventional views, a pathogen is defined as a microbe which possesses genetically determined factors that when expressed will result in diseases in susceptible individuals. However, BV does not follow the traditional route of definition for pathogens since it does not conform to Koch’s postulates; no single agent was noted to reliably cause the disease when inoculated into a healthy woman and no animal model can be sufficiently developed due to constant adaptation of the bacterial species when it comes to life. Therefore, the traditional orthodoxy of monomicrobial approach proposed by Koch is utterly challenged by the polymicrobial nature of BV.
The main factor that will influence the shift from commensal-predominant ecosystem to BV is pH. Most of the main organisms associated with BV, for instance G. vaginalis, anaerobes and M. hominis proliferate in high vaginal pH (pH > 4.5). Factors associated with this are changes in hormonal level especially during period nearly menstruation, semen, race and other differences in physiologic response.
| Implication to Treatment | ▴Top |
The mainstay of treatment in carcinoma of the cervix is radical radiotherapy and concurrent chemo-radiotherapy. The radiotherapy includes a combination of external beam radiotherapy followed by intracavitary brachytherapy. During external beam radiotherapy a homogenous dose of radiation (usually ∼50 Gy) is delivered using high energy photon to the tumor volume and including lymphatic region. Then intracavitary brachytherapy is delivered in various fractionation (8 Gy in 3#HDR or 30 Gy in 1#LDR) schedules using three-dimensional (3D) conformal treatment planning. During a course of radiotherapy, concurrent systemic chemotherapy is administered using cisplatinum 40 mg/m2 every week for 5 weeks. Addition of chemotherapy helps to improve radiosensitivity of tumor and sterilize micrometastases outside the radiation zone. Exposure of radiation therapy and chemotherapy to the bone marrow and lymphatic region could lead to immunosuppression and depression of leucocytes count. Therefore in presence of bacterial infection in the vaginal canal or endocervix could lead to spread of infection to the pelvic tissue causing pelvic inflammatory diseases. Infection in the pelvic region result in inflammation, tissue edema and vascular compromise. Vascular obstruction could result in hypovascularity of tumor and increasing tumor hypoxia. Hypoxic tumors are radioresistant thus the outcome of radiotherapy treatment could be suboptimal leading to local recurrence. The intensity of chemotherapy also affects on peripheral blood lymphocyte (PBL) population by lowering its level and this will increase in opportunistic infections in patient treated for cancer. Brunvard et al [35] reported decreased in CD4+ count and concurrent pneumocystis pneumonia in two women treated with multi-agent chemotherapy and radiation therapy for breast cancer. Few clinical trials have been studied to evaluate pattern of vaginal bacterial growth during radiotherapy. Gilstrap et al [36] evaluated the vaginal and rectal aerobic bacterial flora among 12 gynecologic cancer patients undergoing pelvic radiotherapy. They isolated 59% and 27% Gram positive cocci (GPC) and Gram negative bacilli (GNB) respectively from the vaginal isolates. For example of GPC was Staphylococcus aureus, and for GNB was Klebsiella pneumonia, which have been isolated from high vaginal swab (HVS) (Fig. 1). There was no change in the bacterial count post radiotherapy [36]. Similar study by Gordon et al [37] also did not find any change in the microbial flora before, during and after pelvic radiotherapy. However, in individual patients over 50% microorganisms found in initial culture were no longer present at the completion of radiotherapy.
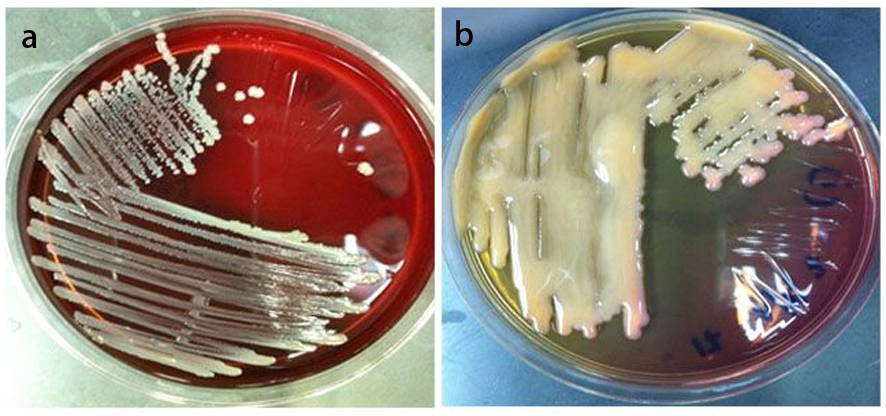 Click for large image | Figure 1. a) Staphylococcus aureus on blood agar (BA), isolated from High Vaginal Swab (HVS) sample, taken from cervical cancer patient. b) Klebsiella pneumonia on MacConkey agar (Mac), isolated from HVS sample, taken from cervical cancer patient. |
A study compared the bacteriologic outcome of three groups of patients on water (22), povidone-iodine (22) and lactic acid vaginal douche. The microbiologic evaluation was preformed on day-0, day-20 Gy and day-40 Gy of treatment. Klebsiella spp., Staphylococcus spp., Pseudomonas pyocynae and Streptococcus fecalis were grown most frequently. The maximum suppression of bacterial growth was encountered in lactic acid vaginal douche group compared to povidone-iodine or water douche only [38]. Bialas et al [39] from Nottingham General Hospital evaluated urinary tract infection before and during pelvic radiotherapy among 172 patients without urinary instrumentation. They observed 17% bacterial infection at the outset and additional 17% during pelvic radiotherapy thus emphasize the influence of radiotherapy in spread of urinary tract infection. Thus, clinicians use empiric antibiotics for prevention of pelvic infection during a course of pelvic radiotherapy in carcinoma cervix. A prospective study from India showed 75% bacterial infection rate and resistance to ciprofloxacillin (38%), levofloxacillin (32%) and cephalosporine (22%) respectively [40]. Therefore, authors recommend use of antibiotics based on microbiologic profile rather than on empiric basis to avoid antibiotic resistance.
| Conclusion | ▴Top |
The association of BV in cervical cancer among women worldwide requires a compulsory screening and treatment in different clinical conditions. Further researches on BV focus pathogenesis and treatment might be important to expand our knowledge on BV and its association with cervical cancer.
| References | ▴Top |
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74-108.
doi pubmed - US Cancer Statistics Working Group. United States Cancer Statistics: 2004 Incidence and Mortality. Atlanta, Ga: Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. 2007.
- Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244-265.
doi pubmed - Castellsague X, Bosch FX, Munoz N. Environmental co-factors in HPV carcinogenesis. Virus Res. 2002;89(2):191-199.
doi - Hawes SE, Kiviat NB. Are genital infections and inflammation cofactors in the pathogenesis of invasive cervical cancer? J Natl Cancer Inst. 2002;94(21):1592-1593.
doi - Georgijevic A, Cjukic-Ivancevic S, Bujko M. [Bacterial vaginosis. Epidemiology and risk factors]. Srp Arh Celok Lek. 2000;128(1-2):29-33.
pubmed - EvyGillet, Joris F, A Meys, Hans Verstraelen et al. Association between Bacterial Vaginosis and Cervical Intraepithelial Neoplasia: Systematic Review and Meta-Analysis. BMC Infectious Disease. 2011;1-9.
pubmed - Verstraelen H, Verhelst R. Bacterial vaginosis: an update on diagnosis and treatment. Expert Rev Anti Infect Ther. 2009;7(9):1109-1124.
doi pubmed - Milena M, Gordana R, Branislava K, Snezana A, Milos S, Vesna M. Complications associated with bacterial vaginosis. Acta Fac. Med. Naiss. 2005;22(4):161-165.
- Sweet RL. Gynecologic conditions and bacterial vaginosis: implications for the non-pregnant patient. Infect Dis Obstet Gynecol. 2000;8(3-4):184-190.
pubmed - Behbakht K, Friedman J, Heimler I, Aroutcheva A, Simoes J, Faro S. Role of the vaginal microbiological ecosystem and cytokine profile in the promotion of cervical dysplasia: a case-control study. Infect Dis Obstet Gynecol. 2002;10(4):181-186.
doi pubmed - Pavic N. Is there a local production of nitrosamines by the vaginal microflora in anaerobic vaginosis/trichomoniasis? Med Hypotheses. 1984;15(4):433-436.
doi - Sexually transmitted diseases treatment guidelines 2002. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51(RR-6):1-78.
- Platz-Christensen JJ, Sundstrom E, Larsson PG. Bacterial vaginosis and cervical intraepithelial neoplasia. Acta Obstet Gynecol Scand. 1994;73(7):586-588.
doi pubmed - Peters N, Van Leeuwen AM, Pieters WJ, Hollema H, Quint WG, Burger MP. Bacterial vaginosis is not important in the etiology of cervical neoplasia: a survey on women with dyskaryotic smears. Sex Transm Dis. 1995;22(5):296-302.
doi pubmed - Callahan DB, Weinberg M, Gunn RA. Bacterial vaginosis in pregnancy: diagnosis and treatment practices of physicians in San Diego, California, 1999. Sex Transm Dis. 2003;30(8):645-649.
doi pubmed - Spiegel CA, Amsel R, Eschenbach D, Schoenknecht F, Holmes KK. Anaerobic bacteria in nonspecific vaginitis. N Engl J Med. 1980;303(11):601-607.
doi pubmed - Gravett MG, Nelson HP, DeRouen T, Critchlow C, Eschenbach DA, Holmes KK. Independent associations of bacterial vaginosis and Chlamydia trachomatis infection with adverse pregnancy outcome. JAMA. 1986;256(14):1899-1903.
doi pubmed - Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988;158(4):819-828.
doi - Discacciati MG, Simoes JA, Lopes ES, Silva SM, Montemor EB, Rabelo-Santos SH, Westin MC. Is bacterial vaginosis associated with squamous intraepithelial lesion of the uterine cervix? Diagn Cytopathol. 2006;34(5):323-325.
doi pubmed - Mikamo H, Sato Y, Hayasaki Y, Kawazoe K, Izumi K, Ito K, Yamada Y, et al. Intravaginal bacterial flora in patients with uterine cervical cancer. High incidence of detection of Gardnerella vaginalis. J Infect Chemother. 1999;5(2):82-85.
doi pubmed - Sikstrom B, Hellberg D, Nilsson S, Kallings I, Mardh PA. Gynecological symptoms and vaginal wet smear findings in women with cervical human papillomavirus infection. Gynecol Obstet Invest. 1997;43(1):49-52.
doi pubmed - Castle PE, Hillier SL, Rabe LK, Hildesheim A, Herrero R, Bratti MC, Sherman ME, et al. An association of cervical inflammation with high-grade cervical neoplasia in women infected with oncogenic human papillomavirus (HPV). Cancer Epidemiol Biomarkers Prev. 2001;10(10):1021-1027.
pubmed - Mao C, Hughes JP, Kiviat N, Kuypers J, Lee SK, Adam DE, Koutsky LA. Clinical findings among young women with genital human papillomavirus infection. Am J Obstet Gynecol. 2003;188(3):677-684.
doi pubmed - Boyle DC, Barton SE, Uthayakumar S, Hay PE, Pollock JW, Steer PJ, Smith JR. Is bacterial vaginosis associated with cervical intraepithelial neoplasia? Int J Gynecol Cancer. 2003;13(2):159-163.
doi pubmed - da Silva CS, Adad SJ, Hazarabedian de Souza MA, Macedo Barcelos AC, Sarreta Terra AP, Murta EF. Increased frequency of bacterial vaginosis and Chlamydia trachomatis in pregnant women with human papillomavirus infection. Gynecol Obstet Invest. 2004;58(4):189-193.
doi pubmed - Watts DH, Fazzari M, Minkoff H, Hillier SL, Sha B, Glesby M, Levine AM, et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis. 2005;191(7):1129-1139.
doi pubmed - Samoff E, Koumans EH, Markowitz LE, Sternberg M, Sawyer MK, Swan D, Papp JR, et al. Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents. Am J Epidemiol. 2005;162(7):668-675.
doi pubmed - Figueiredo PG, Sarian LO, Tambascia JK, Simoes JA, Rabelo-Santos SH, Discacciati MG, Derchain S. Increased detection of clue cells in smears from cervical intraepithelial lesions with reduced expression of COX-2. Diagn Cytopathol. 2008;36(10):705-709.
doi pubmed - Verteramo R, Pierangeli A, Mancini E, Calzolari E, Bucci M, Osborn J, Nicosia R, et al. Human Papillomaviruses and genital co-infections in gynaecological outpatients. BMC Infect Dis. 2009;9:16.
doi pubmed - Nam KH, Kim YT, Kim SR, Kim SW, Kim JW, Lee MK, Nam EJ, et al. Association between bacterial vaginosis and cervical intraepithelial neoplasia. J Gynecol Oncol. 2009;20(1):39-43.
doi pubmed - Rahkola P, Mikkola TS, Ylikorkala O, Vaisanen-Tommiska M. Association between high risk papillomavirus DNA and nitric oxide release in the human uterine cervix. Gynecol Oncol. 2009;114(2):323-326.
doi pubmed - Engberts MK, Vermeulen CF, Verbruggen BS, van Haaften M, Boon ME, Heintz AP. Candida and squamous (pre)neoplasia of immigrants and Dutch women as established in population-based cervical screening. Int J Gynecol Cancer. 2006;16(4):1596-1600.
doi pubmed - Sha BE, Chen HY, Wang QJ, Zariffard MR, Cohen MH, Spear GT. Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. J Clin Microbiol. 2005;43(9):4607-4612.
doi pubmed - Brunvand MW, Collins C, Livingston RB, Raghu G. Pneumocystis carinii pneumonia associated with profound lymphopenia and abnormal T-lymphocyte subset ratios during treatment for early-stage breast carcinoma. Cancer. 1991;67(9):2407-2409.
doi - Gilstrap LC, 3rd, Gibbs RS, Michel TJ, Hauth JC. Genital aerobic bacterial flora of women receiving radiotherapy for gynecologic malignancy. Gynecol Oncol. 1986;23(1):35-39.
doi - Gordon AN, Martens M, LaPread Y, Faro S. Response of lower genital tract flora to external pelvic irradiation. Gynecol Oncol. 1989;35(2):233-235.
doi - John SS. Comparison of cervical flora in patients with carcinoma cervix on external radiation therapy using water, povidone iodine or lactic acid as douche solution. J Clin Oncol. 2004;22(14S):5093.
- Bialas J, Bessell EM, Sokal M, Slack R. A prospective study of urinary tract infection during pelvic radiotherapy. Radioth Oncol. 1989;16(4):305-309.
doi - Joseph B, Sumathi BG, Jayshree RS. Empirical antibiotics treatment in patients with carcinoma cervix being treated with radiotherapy: are we ignoring antibacterial resiatance? Austral J Cancer. 2005;5(4):205-208.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
