| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 3, Number 1, February 2014, pages 14-21
Predictors of Obstetrical Complications: A Prospective Cohort Study
Medyannikova Irinaa, c, Barinov Sergeya, Gudinova Jeanneb
aDepartment of Obstetrics and Gynecology, Omsk State Medical Academy, Russian Federation
bDepartment of General Hygiene With the Course of Hygiene of Children and Adolescents, Omsk State Medical Academy, Russian Federation
cCorresponding author: Medyannikova Irina, Department of Obstetrics and Gynecology, Omsk State Medical Academy; 12, Lenin St., Omsk, 644043, Russian Federation
Manuscript accepted for publication October 11, 2013
Short title: Predictors of Obstetrical Complications
doi: https://doi.org/10.14740/jcgo202w
| Abstract | ▴Top |
Background: The objective was to identify predictors of obstetrical complications.
Methods: A prospective cohort study, a state-run obstetrical hospital, Omsk, Russia. Out of a total 2,497 pregnant women with a gestation age of less than 12 weeks, 2,356 women with singlet pregnancies were selected. Data at gestation outcome were available for 2,177 women. Medical and social factors were evaluated and blood coagulation screening was performed in women in the first trimester. Ordered logistic regression was used to assess risks of obstetrical complications. The following obstetrical complications were evaluated after 28 weeks of gestation: preeclampsia, abruptio placentae, preterm labor, intrauterine growth restriction and intrauterine hypoxia.
Results: A prognostic model comprised of 21 predictors (P < 0.001) with strong association (0.82) between the studied parameters and the obstetrical complications was obtained. Probability of obstetrical complications (91.1%) was associated with the following parameters in women: age, education, parity, medical history, heredity (family history) and blood coagulation profile. Increased fibrinogen concentrations and reduced thrombin clotting time in the first trimester were associated with severe obstetrical complications late in pregnancy.
Conclusions: Women with late obstetrical complications were found to have increased blood viscosity and an increase in coagulation potential early in pregnancy. The prognostic model obtained may have great significance and requires further validation.
Keywords: Pregnancy; Risk factors; Obstetrical complications; Coagulation impairment; Prognosis
| Introduction | ▴Top |
Preeclampsia, abruptio placentae, preterm labor, intrauterine growth restriction (IUGR) and intrauterine fetal demise currently remain major causes of maternal and perinatal morbidity worldwide [1, 2].
In Russia, various degrees of complications during labor and delivery account for 70.1% of all pregnancies; neonatal morbidity comprises 37.3% [3]. Of all labors, 10.2% are preterm, 4.9% are severe preeclampsia, 2.5% involve obstetrical bleeding, and of every 1,000 births, 10.3 result in perinatal mortality, with 57.2% caused by intrauterine hypoxia and asphyxia.
Placentation in humans is now well-established to be associated with unique vascular remodeling. Defective deep placentation has been associated with a spectrum of complications during pregnancy including preeclampsia, IUGR, preterm labor and abruptio placentae [4, 5]. The high rate of placental lesions found in both hypercoagulable and non-hypercoagulable women with severe complications in these studies may explain the difficulty of finding a difference between these two categories of women, or may reflect a still unknown state of hypercoagulability.
Previous studies investigating risk factors for complications of pregnancy have used birth registries or hospital databases [6, 7], randomized trials with negative results [8, 9] and, in a few studies, prospective general population cohorts comprised of high-risk women [10-12].
Study objective
The objective was to develop an algorithm of early (trimester I) risk assessment of late gestation complications using medical, social and coagulation profile factors as potential predictors.
| Methods | ▴Top |
Participants were recruited during 2010 - 2011 by consecutive, population-based enrollment from pregnant women receiving care from the state-run healthcare institution in Omsk Region of Russia, the “Clinical Maternity Hospital No. 1”. These participants included women who were directly enrolled for prenatal care as outpatients or inpatients or by referral from other healthcare institutions.
In order to prevent skewing of highly prognostic coagulation profile screening results, pregnant women with subcompensated and decompensated extragenital medical conditions, malignancies, acute infections and fertility-drug-induced pregnancies were excluded from the study. Complications of pregnancy that could be caused by other etiologies (for example, infectious, endocrine, anatomical, fetal), including habitual abortion, miscarriages before 28 weeks of gestation, cervical incompetence, developmental genital anomalies, multiple pregnancies, placenta previa, fetal anomalies, vasa previa and perinatal fetal injuries were also not considered.
Pregnant women who participated in the study received prenatal care from 12 outpatient maternity clinics and then delivered at 6 maternity hospitals in the city of Omsk. Blood coagulation studies were done at the laboratory of Clinical Maternity Hospital #1. Baseline was determined at the women’s prenatal care visit at 10 - 12 weeks of gestation. Participants then completed 2 follow-up visits at 22 - 24 weeks and 34 - 36 weeks of gestation. Study participation was completed by the end of neonatal period (28th day from child’s birth).
Clinical criteria of the study were assessed starting from 28 weeks of gestation (Table 1). Women were divided into groups based on the severity of their obstetrical and perinatal complications. Women with complications in the second half of pregnancy comprised the main study group. The control group included women with uncomplicated gestation.
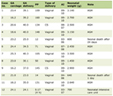 Click to view | Table 1. Clinical Criteria of the Study |
To assess the risk of complications in late pregnancy, 90 potential factors present in the first trimester (80 medical and social factors and 10 laboratory parameters of blood) were considered as potential predictors. The following potential risk factors for obstetrical complications were studied: 1) biosocial factors: age, body mass index, parity, marital status, education, occupation, and economic status; 2) behavioral characteristics: smoking status, dietary habits, and water consumption; 3) heredity (family history): cardiovascular disease, obesity, diabetes mellitus, and bronchial asthma; 4) medical history: diseases of the endocrine, nervous, and cardiovascular systems, infectious diseases, psychiatric conditions, tumors, diseases of eyes, ears, skin, and respiratory organs, and digestive, musculoskeletal and genitourinary diseases; 5) pharmacologic history: estrogen use before and progestin use during pregnancy; 6) family planning care (preconception care); 7) prenatal psychological care (classes for future parents); 8) peripheral complete blood count: hemoglobin, red blood cell (RBC) and white blood cell (WBC) counts, and erythrocyte sedimentation rate (ESR); 9) blood coagulation profile: platelet count, activated partial thromboplastin time (aPTT), prothrombin time (PT), thrombin time, fibrinogen and fibrin split products (soluble fibrin monomers).
The needed sample size was determined based on the sample share [13, 14]. The smallest sample share was accepted to be an average rate of placental abruption (1.2%) in the studied territory; 2,100 pregnant women comprised the smallest sample size. The final sample size, 2,400 participants, was increased by 15% to account for possible loss of participants to follow-up (withdrawals, exclusion criteria).
Statistical analysis was performed using SAS 9.2 software package. The analyzed data included only cases with complete sets of all studied parameters. The critical value of statistical significance for null hypothesis testing was taken to be 0.05 or 0.1. In case the significance level exceeded the critical value, the null hypothesis was accepted to be true. Associations between pairs of qualitative parameters were analyzed using cross-tabulation, odds ratios (OR), with 2-sided 95% confidence intervals (CI) evaluating the value of Neyman-Pearson criterion (χ2).
In order to evaluate the risks of development of late gestational complications, ordered logistic regression method with stepwise algorithms of inclusion and exclusion of predictors was used [15, 16]. As a dependent variable, the parameter “gestation outcome” was used with four gradations: “no complications”, “mild complications”, “moderate complications” and “severe complications”. Relative contribution of predictors is expressed by the Wald statistics value (χ2) and standardized coefficient of regression (standardized estimate). As the prognosis criterion, the percentage of correct (concordant) classification, the value of Somers-D coefficient, was used. About 100 equations of logistic regression were obtained and the equations with the highest values (> 80%) of the parameter were selected.
| Results | ▴Top |
The coverage of the studied population of pregnant women of the city of Omsk comprised 15%. The study sample consisted of 2,356 women with singlet pregnancy before 12 weeks of gestation. The study was completed by 2,212 women, with data available at the end of the study for 2,177 women (Table 2). Gestation-related complications were evaluated after 28 weeks of pregnancy including: preeclampsia, placental abruption, preterm labor, IUGR and intrauterine hypoxia (Table 3).
 Click to view | Table 2. Inclusion/Exclusion Into the Study |
 Click to view | Table 3. Obstetrical and Perinatal Complications in the Study Cohort |
The control group (N = 493) was comprised of women with only physiological changes during pregnancy. The main study group (N = 1,684) included cases with gestation complications of mild (N = 1,165), moderate (N = 340) and severe (N = 179) degrees of severity.
Severe outcomes of gestation were observed in 8.2% (179) of the study participants. Of this group, 0.9% (20) had abruptio placentae, 4.8% (105) had severe preeclampsia, 3.4% (73) went into preterm labor at 28 - 31 weeks, 3.8% (82) experienced IUGR, with body weight < 10 percentile at birth, 4.0% (88) had to have ventilator respiratory support for the baby and out of 1,000 live births, 7.4 experienced perinatal mortality.
The quantitative parameters of the cohort are shown in Table 4.
 Click to view | Table 4. Major Quantitative Parameters in the Study Cohort |
Medical and social parameters of women were significantly different in the groups of physiological and complicated course of the gestation (Supplementary 1).
Unmarried women (OR = 1.6; CI = 1.2 - 2.2; P = 0.002), unemployed homemakers (OR = 1.8; CI = 1.4 - 2.2; P < 0.001) and those who assessed their economical status as being low (OR = 2.0; CI = 1.4 - 2.8; P < 0.001) were more commonly found in the main study group.
Also implicated in the increased risk of unfavorable gestation outcomes of varying degrees of severity were the lack of preconception care (OR = 1.5; CI = 1.2 - 2.1; P = 0.005), including supplementation with folic acid (400 µg/day), tocoferol (10 IU/day), potassium iodide (100 µg/day) and insufficient prenatal psychological preparation (OR = 4.4; CI = 3.6 - 5.5; P < 0.001).
Women’s low body weight (OR = 2.8; CI = 1.9 - 4.2; P < 0.001) and obesity (OR = 1.7; CI = 1.2 - 2.4; P = 0.007) increased the risk of late gestation complications. Pregnant women with less than 1 L consumption of liquids per day (OR = 2.6; CI = 1.9 - 3.6; P < 0.001) and those whose daily food intake included a prevalence of grain-derived products (OR = 2.4; CI = 1.8 - 3.2; P < 0.001) had a higher risk of the conditions studied.
History of diabetes mellitus (OR = 14.3; CI = 7.1 - 29.1; P < 0.001) and bronchial asthma (OR = 8.8; CI = 4.5 - 17.2; P < 0.001) in first and second degree relatives also increased the risk of the studied conditions.
At least one chronic disease was present in histories of 75.8% (1,650) of all women, 49.9% (246) in the control group and 83.4% (1,404) in the main study group. High rates of chronic somatic conditions were found in the study cohort with differences between study groups being significant for all ICD-10 classes of diseases: Class I - Infectious and parasitic diseases: other specified predominantly sexually transmitted diseases (A63.8), herpesviral infection (B00.9); Class II - Neoplasms: hemangioma (D18.0), diffuse cystic mastopathy (N60.1), leiomyoma or uterus (D25.9), other benign neoplasm of cervix uteri (D26.0), benign neoplasm of ovary (D27); Class III - Diseases of the blood: iron-deficiency anemia (D50.9); Class IV - Endocrine diseases: subclinical iodine-deficiency hypothyroidism (E02), nutritional deficiency (E63.9), obesity (E66.9); Class V - Mental and Behavioral Disorders: somatoform autonomic dysfunction (F45.3); Class VI - Diseases of the nervous system: migraine (G43.9); Class VII - Diseases of the eye: myopia (H52.1); Class VIII - Diseases of the ear: otitis media (H66.9); Class IX - Diseases of the circulatory system: essential hypertension (I10), cardiac arrhythmia (I49.9), asymptomatic varicose veins of lower extremities (I83.9), hemorrhoids (I84.9), pelvic varices (I86.2), vulval varices (I86.3), hypotension (I95.9); Class X - Diseases of the respiratory system: chronic nasopharyngitis (J31.1), chronic tonsillitis (J35.0), chronic bronchitis (J41.0), status asthmaticus (J46); Class XI - Diseases of the digestive system: chronic gastritis (K29.5), gastroduodenitis (K29.9), constipation (K59.0), chronic cholecystitis (K81.1), spasm of sphincter of Oddi (K83.4); Class XII - Diseases of the skin: allergic urticaria (L50.0); Class XIII - Diseases of the musculoskeletal system: seronegative rheumatoid arthritis (M06.0), scoliosis (M41.9), spinal osteochondrosis (M42.9); Class XIV - Diseases of the genitourinary system: chronic pyelonephritis (N11.0), ectopic kidney (Q63.2), chronic interstitial cystitis, (N30.1), chronic salpyngitis and oophoritis (N70.1), inflammatory disease of cervix uteri (N72), chronic vaginitis (N76.1), erosion and ectropion of cervix uteri (N86), pelvic pain (N94), postprocedural pelvic peritoneal adhesions (N99.4).
In women with complications in the second half of pregnancy, baseline hemorheologic dysfunction with activation of endothelial/platelet and blood coagulation systems of hemostasis were revealed (Supplementary 2). Baseline impairments in blood coagulation system in women with pathologic pregnancies (main study group) were as follows: 29% (488) had impairments in the endothelial-platelet interaction; 28% (472) had pathological hypercoagulable state; 23% (387) had chronic disseminated intravascular coagulation (DIC) syndrome; 11% (185) had impairment of fibrinolysis; 9% (152) had isocoagulation phenomena.
Analysis of the prediction value dynamics of the selected predictors was worked through with the indication of percentage of correct prediction at each step. Supplementary 3 incorporates the order of inclusion of predictors that have the greatest significance.
Stepwise selection of variables resulted in the statistically significant model (P < 0.001) consisting of 21 predictors (Supplementary 3). According to this model, concordance of over 90% shows that the studied complications can be predicted in the first trimester with high probability. Somers’ D coefficient (> 0.8) points to a strong association of the model’s predictors with the complications studied.
Marital status, occupation, economical status, dietary habits, water consumption and preconception care were not included in the logistic regression equation (χ2 < 1.0; P > 0.1).
When analyzing the prediction value of the selected predictors, blood coagulation parameters that had the highest standardized coefficient module values were fibrinogen, erythrocyte sedimentation rate (ESR), WBC count and thrombin time. Differences in these parameters were associated with the greatest differences in comparing gestation outcomes between the groups.
According to this model, the severity of unfavorable gestation outcome is determined by the following factors, in order of decreasing prediction value of the predictor: fibrinogen (OR = 10.0; CI = 6.25 - 16.67; P = 0.002), ESR (OR = 1.48; CI = 1.43 - 1.54; P ≤ 0.001), age (OR = 1.12; CI = 1.09 - 1.15; P ≤ 0.001), diseases of circulatory system (OR = 3.33; CI = 2.67 - 4.15; P = 0.010), inadequate prenatal psychological care (OR = 3.42; CI = 2.64 - 4.46; P = 0.008), prothrombin time (OR = 1.79; CI = 1.44 - 2.22; P ≤ 0.001), diseases of the genitourinary system (OR = 1.79; CI = 1.43 - 2.23; P = 0.006), pelvic pain (OR = 1.79; CI = 1.47 - 2.19; P ≤ 0.001), hormone therapy (estrogens use before and progestin use during pregnancy) (OR = 1.22; CI = 1.11 - 1.34; P ≤ 0.001), obesity (OR = 1.45; CI = 1.23 - 1.70; P ≤ 0.001), chronic bronchitis (OR = 1.45; CI = 1.21 - 1.75; P ≤ 0.001), education (OR = 1.283; CI = 1.07 - 1.54; P ≤ 0.001), peptic ulcer disease (OR = 2.38; CI = 1.43 - 3.95; P = 0.001), musculoskeletal system diseases (OR = 1.59; CI = 1.16 - 2.19; P = 0.004), neoplasms (OR = 1.61; CI = 1.15 - 2.25; P ≤ 0.001) and herpes virus infection (OR = 1.32; CI = 1.07 - 1.63; P ≤ 0.001).
Factors that decrease the risk of severe obstetrical complications, in order of decreasing the predictor’s prediction value are: WBC count (OR = 0.39; CI = 0.35 - 0.43; P ≤ 0.001), thrombin time (OR = 0.28; CI = 0.24 - 0.34; P ≤ 0.001), parity (OR = 0.44; CI = 0.32 - 0.59; P ≤ 0.001), uncomplicated heredity (family history) (OR = 0.84; CI = 0.79 - 0.90; P ≤ 0.001) and hemoglobin (OR = 0.98; CI = 0.96 - 0.99; P ≤ 0.001).
| Discussion | ▴Top |
In practice, the ability to predict obstetrical complications using an aggregate of all available information about a woman, both clinical and laboratory, would be of interest to many. However, despite numerous studies, the significance of each of the risk factors remains unknown [17-19]. Blood coagulation disorders comprise an important link in the pathogenesis of obstetrical pathology creating a premorbid background for thrombo-hemorrhagic complications [20, 21]. The origins of placental circulatory impairment lie in the development of generalized microangiopathy and hypercoagulability due to cardiovascular, infectious and septic, immunological, metabolic and genetic factors [22, 23].
In order to assess the risk of development of late complications of gestation, 90 potential risk factors were evaluated in pregnant women in the first trimester as probable predictors including 80 medical and social factors and 10 blood laboratory parameters. Using the method of ordered logistic regression, the following prognostic factors of obstetrical complications related to severe gestation outcomes were determined: age, education, parity, medical history, family history and blood coagulation profile. Marital status, occupation, economic status, dietary habits, drinking water consumption and preconception care were not associated with severity of complications studied.
Of note, despite the great number of potentially explanatory variables, as early as the second step of multiregression analysis, chronic diseases were surpassed by blood coagulation tests by their predictive value. Already in the early stages of development of gestational complications, interactions between parts of the hemostasis system are associated with increased blood viscosity and increased coagulation potential along with inhibition of the fibrinolytic system. With the progression of severity of obstetrical complications observed is an increase in concentration of the main coagulation protein (fibrinogen) and in the aggregate activity of clotting factors that comprise the common final pathway of coagulation cascade (decrease in thrombin time). These changes point to the intensification of intravascular blood coagulation.
Previous studies investigating risk factors for complications of pregnancy have used birth registries or hospital databases [6, 7], randomized trials with negative results (that is, no treatment effect shown) [8, 9] and, in a few studies, prospective cohorts (usually general obstetric populations) designed to investigate outcomes of pregnancy [10, 22]. Consistent with other contemporary studies, the women who developed complications during pregnancy were younger, more obese, and more likely to have lower socioeconomic status [23-25]. Many of the risk factors included in the algorithm presented herein are associated with a similar degree of risk to those previously reported, supporting the potential applicability of the algorithm to other populations.
A recent series of publications reported algorithms to predict complications of pregnancy outcome based on clinical risk factors in a general population comprising high risk women, nulliparous women and low risk women (multiparas with previous uncomplicated pregnancies) [9, 10, 26]. A model is fitted to the population, in which it was developed, using the available candidate predictors [27]. A general antenatal population constructed of subgroups with different risk profiles is difficult to replicate. The importance of population differences is evident in the failure of one proposed algorithm to validate in a high-risk population [28], raising questions as to a more general applicability to other populations of pregnant women. Poor performance on validation might also occur because key predictors are missing from the model. When the list of candidate predictors including strongly predictive factors, such as previous complications during pregnancy, renal disease and chronic hypertension [7, 29], these will take precedence, replacing other factors that might be more relevant to healthy women. In contrast, candidate predictors applicable to other populations of pregnant women were investigated in this study.
Potential measurement errors, such as self-reported family history [30], could have occurred, but as the goal was to develop a prototype algorithm ultimately for clinical use, this limitation was accepted. Principal investigators reviewed outcome data for cases, ensuring accurate diagnosis. A challenge when predicting rare events in prospective cohorts is the relatively low numbers of cases compared with studies based on huge epidemiological databases. While the latter might have a substantially greater number of events, interpretation is restrained by less accurate diagnosis.
Conclusion
The concept of a personalized clinical risk estimate for disease, to which biomarkers can be added, is established in several areas of medicine. The algorithm to predict complications of pregnancy reported here provides the first step toward a personalized risk obstetric score for low risk women. It is inevitable the model will be over-fitted to the study population and external validation of the algorithm in other populations of pregnant women is essential. The algorithm’s performance will be evaluated in the next 2,000 women, with nearly all being recruited in different centers. Validation should also be performed in other study populations of pregnant women.
SD: standard deviation.
Acknowledgments
Statistical analysis was performed at the BIOSTATISTICA Centre (E-mail: leo.biostat@gmail.com) under the leadership of Leonov, Vasiliy Petrovich, PhD, associate professor, Department of Informatics, Tomsk State University.
Contribution to Authorship
Each of the authors has made a significant contribution: Irina Medyannikova - to the organization, data collection, data analysis, preparation and approval of the final version of the article; Sergey Barinov - to the organization, data interpretation, preparation and approval of the article; Jeanne Gudinova - to analysis, data interpretation, preparation and approval of the article.
Disclosure of Interests
The authors deny any conflicting financial or other interests.
Ethical Approval
The study protocol was approved by the local ethical committee during the meeting of the Scientific Council of the State Postgraduate Educational Institution “Omsk State Medical Academy” of the Ministry of Health and Social Development of the Russian Federation (Minutes #10 of December 16, 2010).
After obtaining written and oral information about the subject, goals, risks, advantages and duration of the study, as well as alternative prenatal care, the women signed an informed consent document before their participation in the study.
Funding
The study was funded by the Grant of the President of Russian Federation (МK-163.2011.7).
| References | ▴Top |
- Bouvier-Colle MH, Mohangoo AD, Gissler M, Novak-Antolic Z, Vutuc C, Szamotulska K, Zeitlin J. What about the mothers? An analysis of maternal mortality and morbidity in perinatal health surveillance systems in Europe. BJOG. 2012;119(7):880-889; discussion 890.
doi pubmed - Zwart JJ, Richters JM, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population-based study of 371,000 pregnancies. BJOG. 2008;115(7):842-850.
doi pubmed - Healthcare in Russia. Stat. Sat M: Rosstat, 2001-2009.
- Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93(10):1049-1059.
doi pubmed - Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "Great Obstetrical Syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193-201.
doi pubmed - Hernandez-Diaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255.
doi pubmed - Mostello D, Catlin TK, Roman L, Holcomb WL, Jr., Leet T. Preeclampsia in the parous woman: who is at risk? Am J Obstet Gynecol. 2002;187(2):425-429.
doi pubmed - Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, Goldenberg RL, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1997;177(5):1003-1010.
doi - Yu CK, Smith GC, Papageorghiou AT, Cacho AM, Nicolaides KH. An integrated model for the prediction of preeclampsia using maternal factors and uterine artery Doppler velocimetry in unselected low-risk women. Am J Obstet Gynecol. 2005;193(2):429-436.
doi pubmed - Poon LC, Kametas NA, Pandeva I, Valencia C, Nicolaides KH. Mean arterial pressure at 11(+0) to 13(+6) weeks in the prediction of preeclampsia. Hypertension. 2008;51(4):1027-1033.
doi pubmed - Brantsaeter AL, Haugen M, Samuelsen SO, Torjusen H, Trogstad L, Alexander J, Magnus P, et al. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J Nutr. 2009;139(6):1162-1168.
doi pubmed - Silva LM, Coolman M, Steegers EA, Jaddoe VW, Moll HA, Hofman A, Mackenbach JP, et al. Low socioeconomic status is a risk factor for preeclampsia: the Generation R Study. J Hypertens. 2008;26(6):1200-1208.
doi pubmed - Abalos E, Carroli G, Mackey ME. The tools and techniques of evidence-based medicine. Best Pract Res Clin Obstet Gynaecol. 2005;19(1):15-26.
doi pubmed - Lwanga SK, Lemeshow S. Sample size determination in health studies. Geneva: World Health Organization, 1991.
- Adeleke KA, Adepoju AA. Ordinal Logistic Regression Model: An Application to Pregnancy Outcomes. J Math Stat. 2010;6:279-285.
doi - Hosmer DW Jr., Lemeshow S. Applied logistic regression: 2nd ed. John Wiley & Sons, Inc. 2000, 397.
doi - Laursen M, Johansen C, Hedegaard M. Fear of childbirth and risk for birth complications in nulliparous women in the Danish National Birth Cohort. BJOG. 2009;116(10):1350-1355.
doi pubmed - McDonald SD, Best C, Lam K. The recurrence risk of severe de novo pre-eclampsia in singleton pregnancies: a population-based cohort. BJOG. 2009;116(12):1578-1584.
doi pubmed - Trogstad L, Magnus P, Moffett A, Stoltenberg C. The effect of recurrent miscarriage and infertility on the risk of pre-eclampsia. BJOG. 2009;116(1):108-113.
doi pubmed - Lykke JA, Paidas MJ, Damm P, Triche EW, Kuczynski E, Langhoff-Roos J. Preterm delivery and risk of subsequent cardiovascular morbidity and type-II diabetes in the mother. BJOG. 2010;117(3):274-281.
doi pubmed - Papageorghiou AT, Yu CK, Erasmus IE, Cuckle HS, Nicolaides KH. Assessment of risk for the development of pre-eclampsia by maternal characteristics and uterine artery Doppler. BJOG. 2005;112(6):703-709.
doi pubmed - Anderson NH, McCowan LM, Fyfe EM, Chan EH, Taylor RS, Stewart AW, Dekker GA, et al. The impact of maternal body mass index on the phenotype of pre-eclampsia: a prospective cohort study. BJOG. 2012;119(5):589-595.
doi pubmed - Murphy VE, Namazy JA, Powell H, Schatz M, Chambers C, Attia J, Gibson PG. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG. 2011;118(11):1314-1323.
doi pubmed - Silva LM, Steegers EA, Burdorf A, Jaddoe VW, Arends LR, Hofman A, Mackenbach JP, et al. No midpregnancy fall in diastolic blood pressure in women with a low educational level: the Generation R Study. Hypertension. 2008;52(4):645-651.
doi pubmed - Cnossen JS, Leeflang MM, de Haan EE, Mol BW, van der Post JA, Khan KS, ter Riet G. Accuracy of body mass index in predicting pre-eclampsia: bivariate meta-analysis. BJOG. 2007;114(12):1477-1485.
doi pubmed - Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009;53(5):812-818.
doi pubmed - Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605.
doi pubmed - Herraiz I, Arbues J, Camano I, Gomez-Montes E, Graneras A, Galindo A. Application of a first-trimester prediction model for pre-eclampsia based on uterine arteries and maternal history in high-risk pregnancies. Prenat Diagn. 2009;29(12):1123-1129.
doi pubmed - Poon LC, Kametas NA, Chelemen T, Leal A, Nicolaides KH. Maternal risk factors for hypertensive disorders in pregnancy: a multivariate approach. J Hum Hypertens. 2010;24(2):104-110.
doi pubmed - Klemmensen AK, Olsen SF, Osterdal ML, Tabor A. Validity of preeclampsia-related diagnoses recorded in a national hospital registry and in a postpartum interview of the women. Am J Epidemiol. 2007;166(2):117-124.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
