| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 3, Number 1, February 2014, pages 35-41
The Value of Chromohysteroscopy in the Assessment of Postmenopausal Vaginal Bleeding
Yahia M. El-Faissala, b, Ahmed M. Kamela
aObstetrics & Gynecology, Cairo University, Egypt
bCorresponding author: Yahia M. El-Faisssal, 79 Elhussein Street, Dokki, Giza, Egypt
Manuscript accepted for publication October 11, 2013
Short title: Chromo-Hysteroscopy
doi: https://doi.org/10.14740/jcgo209w
| Abstract | ▴Top |
Background: To assess the endometrial changes in postmenopausal patients with vaginal bleeding, using chromohysteroscopy and histopathology, concluding consequently the value of chromohysteroscopy in investigating those patients.
Methods: We included a group of 50 postmenopausal women, presenting with vaginal bleeding, to the outpatient clinic of Cairo University hospital. Transvaginal 2D ultrasonography was performed, followed by hysteroscopy, with chromohysteroscopy performed. Hysteroscopic guided biopsies were obtained from stained areas, then formal curettage done to all uterine walls.
Results: Chromohysteroscopy patterns were either no staining (14%), staining of focal area(s) (76%) or staining of the whole endometrium (10%). Statistical significance was detected between endometrial thickness and chromohysteroscopy. We found a sensitivity 93.75%, specificity 27.77%, PPV 69.76%, and NPV 71.42% of chromohysteroscopy for the pathology obtained.
Conclusion: Chromohysteroscopy improves the efficacy of hysteroscopy in targeting endometrial biopsy. It is though labor intensive and yet not standardized. Studies involving different dyes and larger number of participants are warranted. Inter-observer variability is equally important to help standardizing the procedure.
Keywords: Chromohysteroscopy; Postmenopausal bleeding; Endometrial change
| Introduction | ▴Top |
Postmenopausal bleeding is a common gynecologic complaint, representing up to 69% of postmenopausal women referred to gynecological clinics [1]. Approximately 90% of women with endometrial carcinoma report vaginal bleeding as the sole complaint, so this symptom should always be carefully investigated. However, just 10-15% of women with postmenopausal bleeding have endometrial carcinoma [2].
Dilatation and curettage (D&C) was, for long, considered the “gold standard” for the investigation of postmenopausal bleeding [3]. As a blind procedure, it often results in unrepresentative biopsies [4] with a diagnostic failure that varies from 10 to 25% [5], and false negative rates between 2 and 10%. Older reports have revealed that in 60% of women submitted to curettage less than half of the uterine cavity was sampled with the curette and that the source of bleeding was frequently not diagnosed [6].
Diagnostic hysteroscopy has now replaced conventional cervical D&C for the evaluation of the uterine cavity in industrialized countries. Diagnostic hysteroscopy can be regarded as the gold standard for the evaluation of the uterine cavity. Hysteroscopic surgery is a safe and cost-effective diagnostic and therapeutic tool for the investigation and treatment of intrauterine anomalies [7].
Transvaginal ultrasonography with high-resolution transducers allows a good definition of the endometrial-myometrial interface and visualization of the entire endometrial cavity. Transvaginal ultrasonography is a highly sensitive test for detecting endometrial disease, but carries a false negative rate of 8% for endometrial carcinoma. Several reports showed that the combined assessment of endometrial thickness with some morphologic parameters improves the diagnostic accuracy of transvaginal sonography in patients with postmenopausal bleeding [8].
Chromoendoscopy is a widely used technique in gastrointestinal imaging [9]. Over the last decade, endoscopic systems have acquired great power due to high-resolution images owing to charge-coupled device chip technology and narrow band imaging technique [10]. Based on this growing and advanced applications, chromohysteroscopy was first introduced after the study by Kucuk and Safali in 2008, where they combined chromoendoscopy and hysteroscopy as a new avenue to improve the diagnostic value of hysteroscopy in the setting of assisted reproduction. In this work, we studied the application of chromohysteroscopy in the diagnosis of postmenopausal uterine bleeding [11].
Aim of work
The aim of this study is to correlate the changes occurring in the endometrium of postmenopausal patients with vaginal bleeding, using chromohysteroscopy, together with histopathological examination of biopsies taken from that endometrium. Consequently, and upon analyzing such data, we would reach a conclusion concerning the value of adding chromohysteroscopy as a tool to investigate a patient with postmenopausal vaginal bleeding, as well as its actual impact on the diagnosis.
| Materials and Methods | ▴Top |
A group of consecutive 50 postmenopausal women (no menstrual periods for 12 consecutive months and no other biological nor physiological cause could be identified), presenting with vaginal bleeding, to the outpatient clinic of Cairo University Hospital (Kasr El Eini University Hospital) were included in this study from January 2012 till September 2012. The study has been approved by the internal review board and the Ethical Committee of the Faculty of Medicine, Cairo University.
The patients were properly assessed according to the department’s protocol; for those who were included in the study, a written informed consent of the hysteroscopy procedure was signed by each, including verbal explanation addressing all the details of the procedure of chromohysteroscopy. Transvaginal two-dimensional (2D) ultrasonography, using a GE Voluson E8 ultrasound system (GE Healthcare Ultrasound, Milwaukee, WI, USA) equipped with a 6 - 12 MHz 3D endovaginal probe, was then performed for each, including visual evaluation of uterine dimensions, endometrial thickness and pattern (homogeneity and echogenicity), regularity of the endometrial-myometrial border and fluid in the cavity.
These investigations were done to the patients at the time of their primary visit. They were then scheduled for diagnostic hysteroscopy, during which chromohysteroscopy was performed. A TROPHYSCOPE™ - CAMPO Compact Hysteroscope (Karl Storz®, Tuttningen, Germany), with continuous flow sheath, 5 Fr working channel was used. Distilled water (H2O) was used for uterine distension, with an insufflator that maintained a 100 - 150 mmHg pressure in the uterine cavity. Cervical dilatation was not needed in most of the cases. Those who needed cervical dilatation, were scheduled for diagnostic hysteroscopy under general anesthesia, during which cervical dilatation was performed.
During the setting for chromohysteroscopy, 5 - 10 mL of 2% solution of “methylene blue” (methylthioninium chloride; C16H18N3SCl) (Muby Chemicals, Chinchbunder, Mumbai, India) were instilled into the uterine cavity using a disposable sterile 20 mL plastic syringe connected to the inflow port of the hysteroscope. Uterine distension with distilled water was then resumed for one whole minute, in order to distribute and flush the dye. The patterns of staining of the endometrium were then noted, and were allocated, for statistical convenience, to be one of either:
1) No staining: When no part of the endometrium retains the dye upon examining with the hysteroscope for at least 5 min.
2) Staining of focal area(s) of the endometrium: When a single or multiple localized areas of the endometrium appear more darkly stained than the remainder of the endometrium, following examining with the hysteroscope for at least 5 min.
3) Staining of the whole endometrium: When the entire surface of the endometrium appears homogenously stained with the dye following examining with the hysteroscope for at least 5 min.
Tissue samples were then obtained from stained areas under hysteroscopic guidance. In cases where there is no staining of the endometrium, random endometrial biopsies were taken from the anterior and posterior walls of the uterus, as well as the uterine fundus.
The biopsies obtained were sent to the Pathology Department of Cairo University, for routine histopathological examination. The results were recorded, and then compared with, and correlated to the hysteroscopy findings.
According to the findings, each patient was properly managed according to the institute’s policy.
Statistical methods
Data were analyzed using SPSSwin statistical package version 15 (IBM, Armonk, NY, USA). Numerical data were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. Chi-square test (Fisher’s exact test) was used to examine the relation between qualitative variables. For quantitative data, comparison between two groups was done using Mann-Whitney test (non-parametric t-test). Comparison between more than two groups was done using Kruskal-Wallis test (non-parametric ANOVA), and then post hoc “Schefe test” on rank of variables was used for pair-wise comparison. Pearson’s correlation was used to test relation between numerical variables. A P value < 0.05 was considered significant.
| Results and Discussion | ▴Top |
The patients’ age ranged from 42 to 72 years, with an average mean of 57.5 years. While the parity ranged from 1 to 6 with an average of 3.4 times (Table 1).
 Click to view | Table 1. Age Distribution and Parity of Patients Included in the Study |
Endometrial thickness in this study was estimated by performing the measurement between the two basal layers of the anterior and posterior uterine walls. The poorly reflective layer surrounding the highly reflective endometrium was not included in the measurement. When the endometrial layers were separated with fluid, both layers were measured and the sum was recorded. This is in accordance with the method of measurement adopted in previous studies such as that of Van Den Bosch et al [12] and Sousa et al [2]. However endometrial thickness has been measured in different ways by other authors, such as Fleischer et al [13] who measured the cavity whole through and divided this into two as a measure of single layer endometrial thickness. Nasri and Coast [14] estimated the measure of the endometrium single layer and excluded distension of fluid. Smith et al [15] also recorded a single layer measurement. The endometrial thickness data are shown in Table 2. It is to be noted that the mean values, as well as the distribution of individual measurements, could not yield a cut-off limit for endometrial thickness to identify the pathological endometrium from the normal. This fact is explained by observing the ranges of thickness; the smallest endometrial thickness was 3.0 mm. The difference in endometrial thickness in relation to pathology obtained through chromohysteroscopy revealed a mean of 4.2 mm with a minimum of 3.0 mm in cases with atrophy, a range between 5.0 and 12 mm in endometritis; a mean of 16.5 mm in endometrial hyperplasia with a maximum of 23.0 mm. Cases with endometrial cancer showed a mean endometrial thickness of 25.3 mm, while those with bleeding and non-significant endometrial pathology had a range between 5.0 and 13.0 mm. It should be stated, however, that based on these results, the estimation of endometrial thickness in postmenopausal women could provide a good screening method, helping to predict those women who are liable to experience postmenopausal bleeding.
 Click to view | Table 2. The Correlation Between Endometrial Thickness, Histopathological Diagnosis and Pattern of Subendometrial Perfusion Obtained by Power Doppler Endometrial |
When using the Kruskal-Wallis test to compare the pathology obtained from either fractional curettage or chromohysteroscopy to the endometrial thickness, as shown in Table 2, a P value of < 0.001 was found giving a positive statistical significance.
Chromohysteroscopy
As chromohysteroscopy is a new novel technique in obtaining a targeted endometrial biopsy, the stain used and the method of instillation of the dye and data interpretation were adapted from the study by Kucuk and Deveci [16]. The staining pattern was divided as previously described.
The data gathered showed 76% with focal staining, 14% with no stain and 10% with the whole endometrium stained (Fig. 1). The samples obtained were sent for routine histopathological examination which revealed atrophy in 28%, endometritis in 16%, endometrial hyperplasia in 12%, cancer in 8% and no endometrial pathology (NEP) in 36 % of cases.
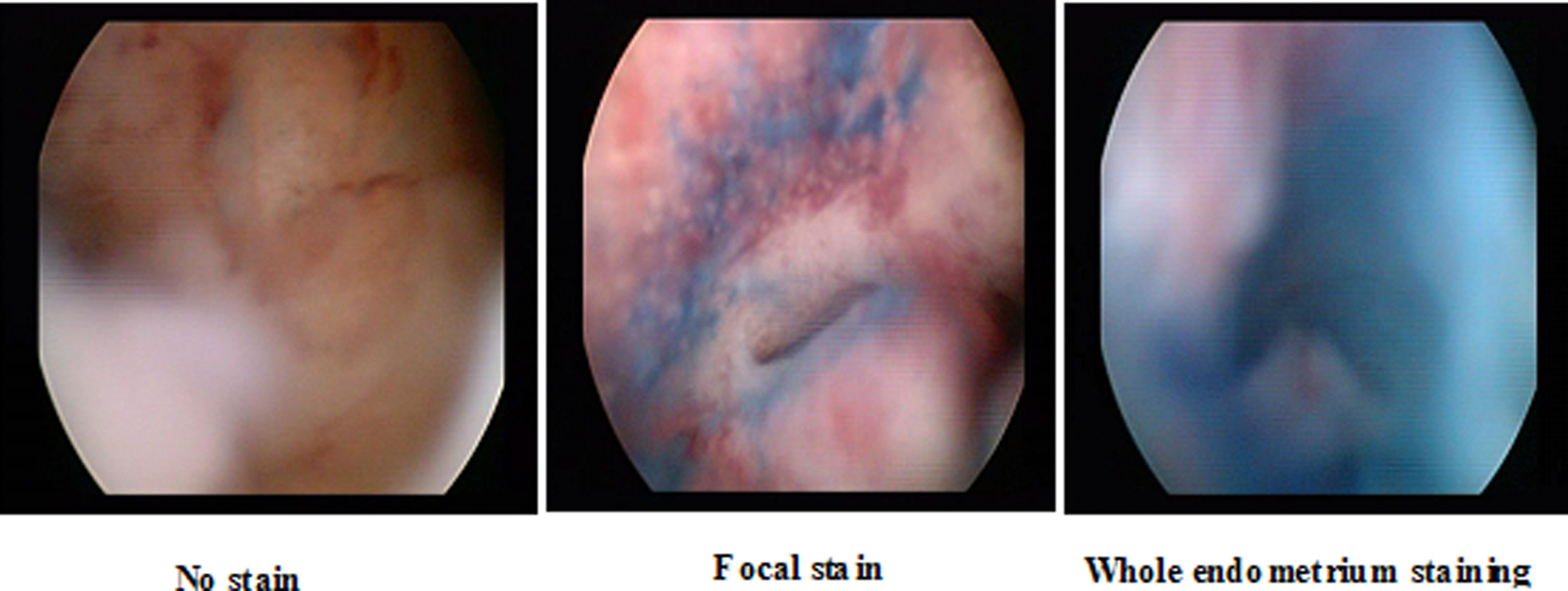 Click for large image | Figure 1. Different patterns of staining at chromohysteroscopy. |
Staining pattern revealed that out of 14 cases of atrophy, 12 showed a focal staining pattern and in the remaining two cases, there was no staining of the endometrium. However, endometritis showed five cases of focal staining and three cases with the whole endometrium stained. All cases of endometrial hyperplasia showed focal staining, while endometrial carcinoma showed focal staining in half of the cases and the other half showed staining of the whole endometrium. Cases with non-significant endometrial pathology showed a focal staining pattern in 13 out of the 18 cases, with the rest showing no staining pattern (Table 3). Chromohysteroscopy led to the diagnosis of three more cases of endometritis and two more cases of focal endometrial hyperplasia, compared to the standard blind fractional curettage. The difference in positive results between fractional curettage and chromohysteroscopy was statistically significant (Table 4).
 Click to view | Table 3. Different Chromohysteroscopy Patterns With Variable Pathology Results |
 Click to view | Table 4. Comparison Between the Different Pathology Results Sampled Through Chromohysteroscopy and Ordinary Fractional Curettage |
Kucuk and Deveci found two extra cases of endometritis and one more case of endometrial hyperplasia when using chromohysteroscopy technique compared to non-targeted endometrial biopsy. Both the results of our study and their study showed greater accuracy in detecting pathological changes in the endometrium. The difference between the results of biopsy by chromohysteroscopy and fractional curettage was statistically significant in our study. They never commented on the statistical significance of their results, probably due to the small number of patients included in their study (22 patients). Another more recent study by Mansour and Mohamed [17] showed that diffuse light staining does not match well with histopathology, so it cannot be relied upon to prove or exclude chronic endometritis. On the other hand, focal dark staining showed sensitivity (70%), specificity (80.8%), positive predictive value (PPV) (43.7%) and negative predictive value (NPV) (92.6%). The result of focal dark staining matched well with the results of histopathology. They consequently concluded that focal dark staining can be used to prove or exclude chronic endometritis.
The sensitivity of chromohysteroscopy technique was 93.75%, specificity was 27.77%, PPV was 69.76% and NPV was 71.42%.
When calculating the sensitivity, specificity, positive and negative predictive values of chromohysteroscopy for the pathology obtained (where positive results = stain (focal and whole endometrium), negative results = no stain, true positive = atrophy, endometritis, endometrial hyperplasia and cancer … with stain, true negative = NEP with no stain, false positive = NEP with stain and false negative = atrophy, endometritis, endometrial hyperplasia and cancer … with no stain), we found a sensitivity of 93.75%, specificity of 27.77%, PPV of 69.76% and NPV of 71.42%. The low specificity recorded in our study is the result of the cases with NEP becoming stained during the procedure; our study did not take into account the varying degrees of stain depth, namely, we did not differentiate between dark and light staining of the endometrium as did the study by Mansour and Mohamed; however, they did not present any criteria identifying the degrees of staining nor the threshold at which the dyeing process was considered whether light or dark apart from the naked eye appreciation of the degree of color. We found such judgment non-standardized and poorly reproducible.
Conclusions and recommendations
In conclusion, chromohysteroscopy improves the efficacy of hysteroscopy in targeting endometrial biopsy. Our study implies endometrial staining with methylene blue dye improves the diagnostic value and provides a method for guided biopsy to diagnose endometrial pathology during hysteroscopy in the absence of macroscopic abnormalities. Chromohysteroscopy seems to be a candidate for implementation in surveillance programs for high risk patients, but it is a labor intensive and time consuming technique, which so far has prevented its wide spread use. Future studies involving chromohysteroscopy should focus on its value in clinical practice by average experienced endoscopists. Other studies should be carried out with different dyes, as different dyes have different staining properties, and some will be more suited for infection, while others more suited for dysplasia and neoplasia. More extensive research is still warranted in order to establish the previous suggestions. Studies that include a larger number of patients and long-term follow-up are required to verify the real predictive value of chromohysteroscopy in postmenopausal bleeding, as well as their role in the diagnosis. Lastly, it is of great importance to carry out further studies concerning the inter-observer and the intra-observer reproducibility, and with accumulating results, a standardized protocol could be established.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
| References | ▴Top |
- Tahir MM, Bigrigg MA, Browning JJ, Brookes ST, Smith PA. A randomised controlled trial comparing transvaginal ultrasound, outpatient hysteroscopy and endometrial biopsy with inpatient hysteroscopy and curettage.Br J ObstetGynaecol. 1999;106(12):1259-1264.
doi pubmed - Sousa R, Silvestre M, Almeida e Sousa L, Falcao F, Dias I, Silva T, De Oliveira C, et al. Transvaginal ultrasonography and hysteroscopy in postmenopausal bleeding: a prospective study. ActaObstetGynecol Scand. 2001;80(9):856-862.
pubmed - Buyuk E, Durmusoglu F, Erenus M, Karakoc B. Endometrial disease diagnosed by transvaginal ultrasound and dilatation and curettage. ActaObstetGynecol Scand. 1999;78(5):419-422.
doi pubmed - Loverro G, Bettocchi S, Cormio G, Nicolardi V, Greco P, Vimercati A, Selvaggi L. Transvaginalsonography and hysteroscopy in postmenopausal uterine bleeding. Maturitas. 1999;33(2):139-144.
doi - Nagele F, O'Connor H, Davies A, Badawy A, Mohamed H, Magos A. 2500 Outpatient diagnostic hysteroscopies. Obstet Gynecol. 1996;88(1):87-92.
doi - Towbin NA, Gviazda IM, March CM. Office hysteroscopy versus transvaginal ultrasonography in the evaluation of patients with excessive uterine bleeding. Am J Obstet Gynecol. 1996;174(6):1678-1682.
doi - Wieser F, Tempfer C, Kurz C, Nagele F. Hysteroscopy in 2001: a comprehensive review. ActaObstetGynecol Scand. 2001;80(9):773-783.
doi pubmed - Weber G, Merz E, Bahlmann F, Rosch B. Evaluation of different transvaginalsonographic diagnostic parameters in women with postmenopausal bleeding. Ultrasound Obstet Gynecol. 1998;12(4):265-270.
doi pubmed - Sano Y, Muto M, Tajiri H, Ohtsu A, Yoshida S. Optical/digital chromoendoscopy during colonoscopy using narrow-band imaging system. Digestive Endosc. 2005;17:S43-S48.
doi - Dacosta RS, Wilson BC, Marcon NE. New optical technologies for earlier endoscopic diagnosis of premalignant gastrointestinal lesions.J GastroenterolHepatol. 2002;17(Suppl):S85-104.
doi pubmed - Kucuk T, Safali M. "Chromohysteroscopy" for evaluation of endometrium in recurrent in vitro fertilization failure. J Assist Reprod Genet. 2008;25(2-3):79-82.
doi pubmed - Van den Bosch T, Van Schoubroeck D, Ameye L, Van Huffel S, Timmerman D. Ultrasound examination of the endometrium before and after Pipelle endometrial sampling. Ultrasound Obstet Gynecol. 2005;26(3):283-286.
doi pubmed - Fleischer AC, Mendelson EB, Bohm-Velez M, Entman SS. Transvaginal and transabdominalsonography of the endometrium. Semin Ultrasound CT MR. 1988;9(2):81-101.
pubmed - Nasri MN, Coast GJ. Correlation of ultrasound findings and endometrial histopathology in postmenopausal women.Br J ObstetGynaecol. 1989;96(11):1333-1338.
doi - Smith P, Bakos O, Heimer G, Ulmsten U. Transvaginal ultrasound for identifying endometrial abnormality. ActaObstetGynecol Scand. 1991;70(7-8):591-594.
doi pubmed - Kucuk T, Deveci S. Can "chromohysteroscopy" help target endometrial biopsy in postmenopausal bleeding? Eur J GynaecolOncol. 2008;29(2):165-167.
pubmed - Mansour H, Mohamed MA. Value of endometrial dyeing in diagnosis of endometritis in the absence of macroscopic abnormalities during hysteroscopy.Middle East Fertility Society Journal. 2008;16:83-86.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
