| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 4, Number 4, December 2015, pages 297-301
Comparison of Electromyographic Evaluation of Pelvic Floor Muscles in Third Trimester Between Pregnant Women With and Without Urinary Incontinence
Ana Silvia Moccellina, c, Mariana Tirolli Retta, Patricia Driussob
aDepartment of Physiotherapy, Federal University of Sergipe - UFS, Sao Cristovao, Sergipe, Brazil
bDepartment of Physiotherapy, Federal University of Sao Carlos - UFSCar, Sao Carlos, Sao Paulo, Brazil
cCorresponding Author: Ana Silvia Moccellin, Department of Physiotherapy, Federal University of Sergipe - UFS, Rua Claudio Batista - lado par, Santo Antonio, Sao Cristovao, Sergipe, Brazil
Manuscript accepted for publication October 02, 2015
Short title: Pelvic Floor Muscles in Pregnant Women
doi: http://dx.doi.org/10.14740/jcgo361w
| Abstract | ▴Top |
Background: The aim of the study was to compare the pelvic floor muscle function between pregnant women with and without urinary incontinence, in third trimester.
Methods: Fifteen pregnant women with urinary incontinence and 25 pregnant women without urinary incontinence were included in study. The evaluation was conducted in two moments on third trimester: between the 24th and 28th gestational week and between the 34th and 36th gestational week, consisted on electromyographic evaluation of pelvic floor muscles function. Data were tabulated in Excel and statistically analyzed with the Statistica program. A significance level of 5% (P ≤ 0.05) was adopted.
Results: The comparison between the groups showed significant difference, in first evaluation, on the mean and peak values of sustained contraction, and pregnant women with urinary incontinence had the worst values of the electrical signal related to these variables. The intragroup comparison, pregnant women with urinary incontinence had, at the end of pregnancy, higher mean values of the pelvic floor muscles electrical activity during maximal voluntary and sustained contractions, and greater peak value during maximal voluntary contraction.
Conclusions: Pregnant women with urinary incontinence complaint had worse pelvic floor muscles function, compared to those without complaint. Thus, the pelvic floor muscle function is directly related to continence in pregnant women.
Keywords: Pregnancy; Pelvic floor; Urinary incontinence; Electromyography
| Introduction | ▴Top |
During pregnancy, the anatomical and physiological damages that occur in the lower urinary tract can alter its function, inducing voiding symptoms, which tend to become more pronounced as the pregnancy progresses [1, 2]. So these symptoms are more common in the third trimester and may be related to the pressure on the bladder from the fetal head [3].
According to Morkved et al [4], the function of the pelvic floor muscles (PFM), assessed by ultrasonography and perineometer, is better in continent pregnant women compared to primigravidae with urinary incontinence, because these women have less muscle thickness and, therefore, less ability to compress the urethra during a sudden increase in intraabdominal pressure.
However, the evidences are not sufficient to support the hypothesis that the PFM function is directly related to continence in pregnant women, so that a decrease in electrical activity of the PFM is associated with the urinary incontinence complaint. Thus, it becomes necessary to evaluate the function of these muscles during pregnancy, relating it to urinary loss complaint.
Among the assessment methods of PFM function, the electromyography (EMG) stands out because it allows consistently predicting certain clinical variables related to the function of these muscles [5]. This method is able to demonstrate the baseline, the function of phasic and tonic fibers, which provides a complete muscle function assessment [6].
The objective of this study was to compare the PFM function between pregnant women with and without urinary incontinence, in the third trimester.
| Material and Methods | ▴Top |
This is a cross-sectional study conducted from July 2012 to October 2013. The sample size calculation was performed using G* Power 3.1.3 program. The values found in Frederice et al’s [7] study were used as parameter for the electromyographic activity of the PFM during pregnancy. For a power of 0.90 and alpha test error of 5%, 15 patients per group was suggested (pregnant women with and without urinary incontinence).
The study was conducted in two family health units in Aracaju city (SE). While attending prenatal program, the pregnant women were invited to participate at the study by the responsible researcher. Being aware of the study and voluntarily agreeing to participate, the first evaluation was scheduled. All women signed the consentient form.
Inclusion criteria were: pregnant women aged between 18 and 40 years, body mass index (BMI) before pregnancy considered normal, based on the World Health Organization (WHO) concept [8], gestational age between 24 and 28 weeks, low risk, single pregnancy and who were in prenatal care. Exclusion criteria were: risk of abortion, uterine bleeding, previous recurrent abortion, urinary tract infection and/or inflammation, cognitive impairment, illicit drug, smoking and alcohol intake.
The evaluation was conducted in two moments in third trimester: between the 24th and 28th gestational week and between the 34th and 36th gestational week, according to the date of last menstruation [9] and/or the first ultrasound performed during pregnancy [10]. The pre-pregnancy BMI was collected through prenatal care card and gestational BMI was assessed based on Atalah table [11].
The criterion used to define urinary loss included two standardized questions that compose the scale of urinary symptoms of the King Health Questionnaire (KHQ), developed by Kelleher et al [12]. The KHQ is a specific questionnaire for assessing quality of life in incontinent women, being able to assess the perceived impact of urinary incontinence in women’s lives and the measures of its severity. The questions were: “Do you lose urine during physical effort such as coughing, sneezing, running?” and “Do you lose urine when you feel urgency to urinate?” Pregnant women who answered “yes” to at least one of these questions were classified in the group with urinary incontinence complaint and characterized with urinary symptoms.
For the PFM evaluation, the MyoTrac InfinitTM surface EMG was used with the following specifications: converting the original signal to the root mean square value (RMS), band pass filter of 20 - 500 Hz, common mode rejection rate (CMRR) > 130 dB and active electrode impedance of 1,012 GΩ. Data were normalized by the peak value among three maximum voluntary contractions performed [13, 14].
This instrument records the sum of the electrical potentials generated by the muscle fibers depolarization at rest and during voluntary contraction while its amplitude is recorded in microvolts (µV). It is the most accurate method to measure the neuromuscular integrity and can be considered an indirect measure of PFM strength and pressure level during a contraction [15].
The positioning during evaluation was supine with hip and knee flexion, and feet flat. The examiner introduced a vaginal sensor (model AS 9572 Brand Thought Technology Ltd® with surface capture of stainless steel with 27 mm diameter and 69 mm length), lubricated with water soluble gel on vaginal opening. Two reference electrodes were placed on the right anterior superior iliac crest and on the right lateral malleolus. Self-adhesive electrodes were placed on rectus abdominal region for simultaneous measurements of PFM and abdominal muscle activities.
Initially, the volunteer was asked to rest for 15 s to record the basal activity. After this, three maximal voluntary contractions (MVCs), maintained by 2 s, with an interval of 1 min between each one, and three sustained contractions, held for 6 s, with an interval of 1 min between each one were recorded [13].
In order to identify performing Valsalva maneuver and/or simultaneous contraction of the hip and buttocks adductor muscles, instead of isolated PFM contraction, the abdomen and the perineal region were observed during the PFM contraction. When there was accessory muscles contraction, PFM contraction was not recorded.
Data were tabulated in Excel and statistically analyzed with the Statistica program and through descriptive techniques. The Shapiro-Wilk test indicated nonparametric tests. The Wilcoxon matched pairs test was used to compare the intragroup PFM electromyographic activity. The Mann-Whitney test was used for comparison between pregnant women with and without urinary incontinence. The Chi-square test and, when necessary, Fisher’s exact test were used to evaluate the association between qualitative variables. A significance level of 5% (P ≤ 0.05) was adopted. Data are expressed as mean ± standard deviation.
| Results | ▴Top |
The study included 40 pregnant women (15 with urinary incontinence and 25 without urinary incontinence). Table 1 shows the anthropometric characteristics and the gestational age mean in each evaluation.
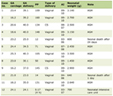 Click to view | Table 1. Anthropometric Characteristics of Pregnant Women With and Without Urinary Incontinence |
Table 2 shows the pregnant women distribution by parity. It is possible to note that for the group with urinary incontinence complaints, there is higher prevalence of secundigravidae of previous vaginal delivery, while in the group without urinary incontinence, the highest proportion is among the primigravidae.
 Click to view | Table 2. Distribution of Parity Characteristics of Pregnant Women With and Without Urinary Incontinence |
All pregnant women with urinary symptoms reported urine loss episodes in the last month previous to the assessment. Among these, 80% (n = 12) reported the onset of symptoms during pregnancy.
Table 3 presents the variables of PFM functional evaluation during the third trimester in both groups. The comparison between the groups showed significant difference, in the first evaluation, on the mean and peak values of sustained contraction, and pregnant women with urinary incontinence had the worst values of the electrical signal related to these variables. For the intragroup comparison, pregnant women with urinary incontinence had, at the end of pregnancy, higher mean values of the PFM electrical activity during MVC and sustained contraction, and greater peak value during MVC. Pregnant women without urinary incontinence had, in the last evaluation, a significant decrease on the PFM mean value at rest.
 Click to view | Table 3. Functional Evaluation of PFM at Rest and During Contractions in Pregnant Women With and Without Urinary Symptoms in the Periods Between 24th and 28th (Evaluation 1) and 34th and 36th (Evaluation 2) Gestational Weeks |
| Discussion | ▴Top |
The urethral closure mechanism is determined by the urethral sphincter tonus, made by smooth and striated skeletal muscles beyond the submucosal vascular elements that contribute to the maintenance of urethral coaptation [16]. During pregnancy, due to anatomical and physiological damages that occur in the pelvic floor, this mechanism may change, such as decrease in recruitment of motor units during a contraction [1, 17]. Our results show that, among pregnant women with urinary incontinence complaint, 80% reported the onset of urinary symptoms during pregnancy, which leads us to believe that the changes which occur during pregnancy may be directly related to possible PFM dysfunctions.
Thereby, due to these alterations, a compensatory mechanism that does not occur in many pregnant women, allowing the bladder pressure to exceed the urethral pressure, is necessary [18]. In this study, it was observed that pregnant women with urinary incontinence showed a pattern of electromyographic activity with values below those presented by pregnant women without complaint. However, in Frederice et al’s [7] study, no association between the degree of muscle strength, MVC and average sustained contraction with the presence of urinary incontinence in pregnant women was observed. The authors claim that urinary incontinence can occur due to changes in the muscles positioning and not by a change in the muscle contraction.
The evaluations were performed at the beginning and the end of the third trimester, because there is evidence that urinary symptoms tend to become more pronounced with increasing gestational age, being most prevalent in the third trimester and may be related to the pressure on bladder exerted by the fetal head [3]. Santos et al [19] reported that the onset of urinary incontinence complaints occurred at 27 gestational weeks on average. Hojberg et al [20] affirmed that there was an increase in the complaints prevalence from the 20th gestational week.
Besides pressure from fetal head, the maternal body mass and the gravid uterus mass cause an overload on the pelvic floor structures [3]. Our results show that, in both groups of pregnant women, there was a significant increase in BMI; however, the values remained within normal limits [11]. Therefore, at the end of pregnancy, as a result of chronic stress, the endopelvic fascia gradually elongated and toned can lose its pelvic organs support and continence maintenance functions, which can trigger urinary symptoms [21].
Maternal age and parity are also indicated as risk factors for the onset of urinary symptoms during pregnancy [22]. One study found that women over 35 years old are more prone to developing PFM disorders, because the physiological aging is accompanied by an increase in density of PFM fiber denervation [23]. Our results show that there was a significant difference in maternal age, with pregnant women without urinary incontinence complaint presenting lower age; however, the two groups presented maternal age below 35 years, which may have diminished the influence of this variable on urinary symptoms.
Regarding parity, in our study, among pregnant women with urinary incontinence, the largest proportion was secundigravidae with previous vaginal delivery. However, there is evidence that urinary symptoms also occur in the first pregnancy [24]. It is noteworthy that 26.7% of pregnant women with urinary incontinence complaint are primigravidae and, among these pregnant women without complaint, 40% are secundigravidae with previous vaginal delivery, suggesting that pregnancy itself may be associated with pelvic floor changes.
Some limitations need to be considered in this study. Although the pregnant women were questioned about the period that began voiding symptoms, their PFM was not evaluated before pregnancy, which could contribute to understanding the relationship between the lowest values of the PFM electromyographic signal and the presence of urinary incontinence. Finally, it is important to highlight the criteria used to define urine loss that can vary between studies and, in this study, included two standardized questions that compose the scale of urinary symptoms of the KHQ.
Conclusion
It can be concluded that pregnant women with urinary incontinence complaint had worse PFM function during MVC and sustained contraction, compared to those without complaint. For urinary continence conservation during pregnancy, it is necessary that the intrinsic and extrinsic sphincter mechanisms are undamaged with adequate urethral closure pressure and bladder neck support [25]. Thus, our hypothesis that the PFM function is directly related to continence in pregnant women has been proven.
Acknowledgement
The authors thank FAPESP for the financial support (FAPESP No. 2011/20904-2) and the Community Care Units from Aracaju’s County Health Department (Aracaju city -SE).
Conflict of Interest
The authors declare no conflict of interest.
| References | ▴Top |
- Chaliha C, Stanton SL. Urological problems in pregnancy. BJU Int. 2002;89(5):469-476.
doi pubmed - van Brummen HJ, Bruinse HW, van der Bom JG, Heintz AP, van der Vaart CH. How do the prevalences of urogenital symptoms change during pregnancy? Neurourol Urodyn. 2006;25(2):135-139.
doi pubmed - Scarpa KP, Herrmann V, Palma PC, Ricetto CL, Morais S. [Prevalence of urinary symptoms in the third trimester of pregnancy]. Rev Assoc Med Bras. 2006;52(3):153-156.
doi pubmed - Morkved S, Salvesen KA, Bo K, Eik-Nes S. Pelvic floor muscle strength and thickness in continent and incontinent nulliparous pregnant women. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(6):384-389; discussion 390.
doi pubmed - Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4-20.
doi - Grape HH, Dedering A, Jonasson AF. Retest reliability of surface electromyography on the pelvic floor muscles. Neurourol Urodyn. 2009;28(5):395-399.
doi pubmed - Frederice CP, Amaral E, Ferreira Nde O. Urinary symptoms and pelvic floor muscle function during the third trimester of pregnancy in nulliparous women. J Obstet Gynaecol Res. 2013;39(1):188-194.
doi pubmed - WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series 854. Geneva: World Health Organization,1995.
- Alexander GR, Tompkins ME, Cornely DA. Gestational age reporting and preterm delivery. Public Health Rep. 1990;105(3):267-275.
pubmed - Rossavik IK, Fishburne JI. Conceptional age, menstrual age, and ultrasound age: a second-trimester comparison of pregnancies of known conception date with pregnancies dated from the last menstrual period. Obstet Gynecol. 1989;73(2):243-249.
pubmed - Atalah E, Castillo C, Castro R, Aldea A. [Proposal of a new standard for the nutritional assessment of pregnant women]. Rev Med Chil. 1997;125(12):1429-1436.
pubmed - Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol. 1997;104(12):1374-1379.
doi pubmed - Soderberg GL, Knutson LM. A guide for use and interpretation of kinesiologic electromyographic data. Phys Ther. 2000;80(5):485-498.
pubmed - Ervilha UF, Duarte M, Amadio AC. Estudos sobre procedimentos de normalizacao do sinal eletromiografico durante o movimento humano. Rev Bras Fisiot. 1998;3(1):15-20.
- Shafik A, Doss S, Asaad S. Etiology of the resting myoelectric activity of the levator ani muscle: physioanatomic study with a new theory. World J Surg. 2003;27(3):309-314.
doi pubmed - Ashton-Miller JA, DeLancey JO. Functional anatomy of the female pelvic floor. Ann N Y Acad Sci. 2007;1101:266-296.
doi pubmed - Herbert J. Pregnancy and childbirth: the effects on pelvic floor muscles. Nurs Times. 2009;105(7):38-41.
pubmed - van Geelen JM, Lemmens WA, Eskes TK, Martin CB, Jr. The urethral pressure profile in pregnancy and after delivery in healthy nulliparous women. Am J Obstet Gynecol. 1982;144(6):636-649.
pubmed - Santos PC, Mendonca D, Alves O, Barbosa AM. [Prevalence and impact of stress urinary incontinence before and during pregnancy]. Acta Med Port. 2006;19(5):349-356.
pubmed - Hojberg KE, Salvig JD, Winslow NA, Lose G, Secher NJ. Urinary incontinence: prevalence and risk factors at 16 weeks of gestation. Br J Obstet Gynaecol. 1999;106(8):842-850.
pubmed - Calguneri M, Bird HA, Wright V. Changes in joint laxity occurring during pregnancy. Ann Rheum Dis. 1982;41:126-128.
doi - Viktrup L, Lose G. The risk of stress incontinence 5 years after first delivery. Am J Obstet Gynecol. 2001;185(1):82-87.
doi pubmed - Fritel X, Fauconnier A, Levet C, Benifla JL. Stress urinary incontinence 4 years after the first delivery: a retrospective cohort survey. Acta Obstet Gynecol Scand. 2004;83(10):941-945.
doi pubmed - Ekstrom A, Altman D, Wiklund I, Larsson C, Andolf E. Planned cesarean section versus planned vaginal delivery: comparison of lower urinary tract symptoms. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):459-465.
doi pubmed - Dietz HP, Clarke B, Herbison P. Bladder neck mobility and urethral closure pressure as predictors of genuine stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13(5):289-293.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
