| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 5, Number 1, March 2016, pages 53-57
An Obstetric Perspective on Functional Bowel Obstruction After Cesarean Section: A Case Series
Kimberley J. Norton-Olda, c, Nicola Yuenb, Mark P. Umstadb
aThe Women’s at Sandringham, The Royal Women’s Hospital, Sandringham, Victoria 3191, Australia
bDepartment of Obstetrics and Gynaecology, The Royal Women’s Hospital, Melbourne, Victoria 3052, Australia
cCorresponding Author: Kimberley Norton-Old, The Women’s at Sandringham, The Royal Women’s Hospital, PO Box 543, Richmond, Victoria 3121, Australia
Manuscript accepted for publication March 01, 2016
Short title: Bowel Obstruction After Cesarean Section
doi: http://dx.doi.org/10.14740/jcgo390w
| Abstract | ▴Top |
Ogilvie’s syndrome (OS) and paralytic ileus are two types of functional bowel obstructions well known in obstetrics. Cesarean section and spinal anesthetic have been reported as etiological factors. OS is rare; however, associated morbidity and mortality rates are high. We report on three patients whose post-cesarean section course was complicated by a functional bowel obstruction. Two of the patients exhibited signs of imminent perforation from cecal dilation and required major surgical intervention. The pathophysiology of OS and paralytic ileus and their respective management are discussed based on a review of the literature. A new approach to the management of functional bowel obstructions after cesarean section is offered from an obstetric perspective. Identification of cecal dilation is the key. We suggest prompt measurement of the cecum with an abdominal X-ray followed by surgical review and intervention in symptomatic patients with a cecal diameter equal to or greater than 10 cm.
Keywords: Acute colonic pseudo-obstruction; Ogilvie’s syndrome; Paralytic ileus; Cesarean section; Cecal perforation
| Introduction | ▴Top |
Two of the most common causes of functional bowel obstruction following cesarean section are paralytic ileus and Ogilvie’s syndrome (OS). One of the most severe sequelae of these etiologies is bowel perforation, with the cecum being the most susceptible [1]. Cecal dilation greater than 10 cm can result in rupture or ischemic perforation of the bowel, which carries a mortality rate of up to 72% [2, 3].
Paralytic ileus occurs from prolonged hypomotility of the gastrointestinal tract secondary to inflammation of intestinal smooth muscle [4, 5]. Moderate to severe cases have been documented in 10-20% of post-cesarean patients [6].
Acute colonic pseudo-obstruction (ACPO) or OS is the clinical syndrome of acute large bowel dilation in the absence of mechanical cause [7]. Although OS is considered rare, there is strong evidence in the medical literature of its occurrence after cesarean section, laparoscopic and orthopedic surgery and after spinal anesthetic [8, 9]. Its pathogenesis remains unknown but it appears to be from an imbalance in the autonomic regulation of colonic motor function resulting in bowel atony and dilation. Conservative and pharmacological therapies are effective, but surgical intervention may be required if there is risk of perforation in order to prevent intra-abdominal sepsis [1]. Vanek and Al-Salti reported that maximal cecal diameter and delay in colonic decompression have a significant direct correlation to mortality [10]. The mortality rate in a healthy patient with no complications and early intervention is about 15% as compared with 36-50% in patients with a perforated or ischemic bowel [10, 11].
Previously, clinical and radiological differentiation of the two conditions directed the course of treatment and early diagnosis of the type of functional bowel obstruction was seen as a crucial step in minimizing patient morbidity. However, we emphasize that ischemic damage of the intestinal wall from excessive dilation, regardless of the type of function bowel obstruction, is the main etiological factor here. For this reason, we propose that the primary predictor of morbidity and mortality outcomes in symptomatic post-cesarean patients is cecal diameter.
In this report, two patients with OS involving acute colonic ischemia with necrosis managed surgically are presented, as is a third case with paralytic ileus which resolves with conservative management. These three cases occurred within the same week, prompting an urgent review.
| Case Reports | ▴Top |
Case 1
A 32-year-old woman, para 1, was admitted to the postnatal ward after emergency cesarean section for failure to progress at term. No intra-operative complications or bowel involvement were documented. On the first postoperative day (POD), her pain was well controlled and she was tolerating oral intake. On examination, she had a soft, non-distended abdomen with audible bowel sounds. She remained clinically well with normal vital signs and began passing flatus on day 2. She then reported mild abdominal distension and constipation despite regular laxatives. She requested discharge on day 3 postoperation without passing a bowel motion.
She represented on day 4 after cesarean section complaining of severe lower abdominal pain and worsening distension despite passing flatus and bowel motions. Physical examination revealed a grossly distended and peritonitic abdomen. Bowel sounds were audible although sluggish. Vital signs remained with normal range; however, she was febrile at 37.8 °C. Laboratory results showed an acute neutrophilia and C-reactive protein (CRP) of 120 mg/L. Abdominal X-rays revealed widespread colonic dilation with no free air (Fig. 1) and subsequent computed tomography (CT) scan confirmed a transition point within the distal colon. A provisional diagnosis of ACPO was made. An emergency diagnostic laparoscopy was performed and converted to laparotomy where necrotic cecum was found and a right hemi-colectomy with anastomosis was performed. The key histological features were focal transmural necrosis and incipient perforation, in keeping with the radiological suggestion of intestinal pseudo-obstruction. Her postoperative course was unremarkable and she was discharged day 6 after laparotomy.
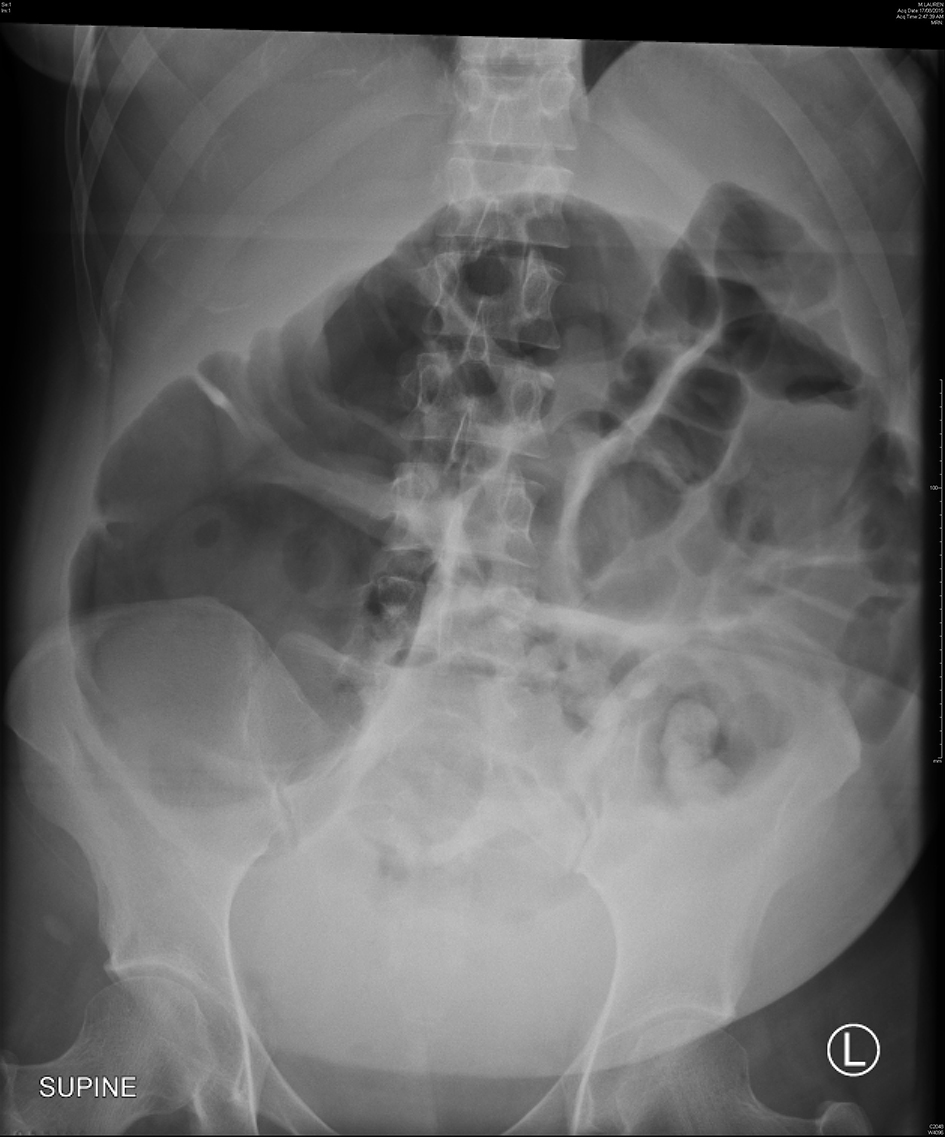 Click for large image | Figure 1. Abdominal X-ray of case 1 showing a dilated colon with the cecum measuring 12.5 cm in diameter. |
Case 2
A 32-year-old, para 2 woman underwent an uncomplicated, elective cesarean section at 39 weeks. On POD 1, she reported worsening lower abdominal pain, non-bilious vomiting and constipation. Physical examination revealed a grossly distended abdomen, which was generally tender with guarding in the right iliac fossa. There was no clinical evidence of peritonitis. Vital signs were normal and laboratory results showed a CRP of 127 mg/L. Plain abdominal radiographs showed gaseous distension of large and small bowel (Fig. 2) and repeated following oral gastrografin. CT imaging showed widespread colonic dilation with no transition point and a maximum cecal diameter of 9.5 cm. The general surgeons reviewed her the same day and diagnosed a postoperative ileus.
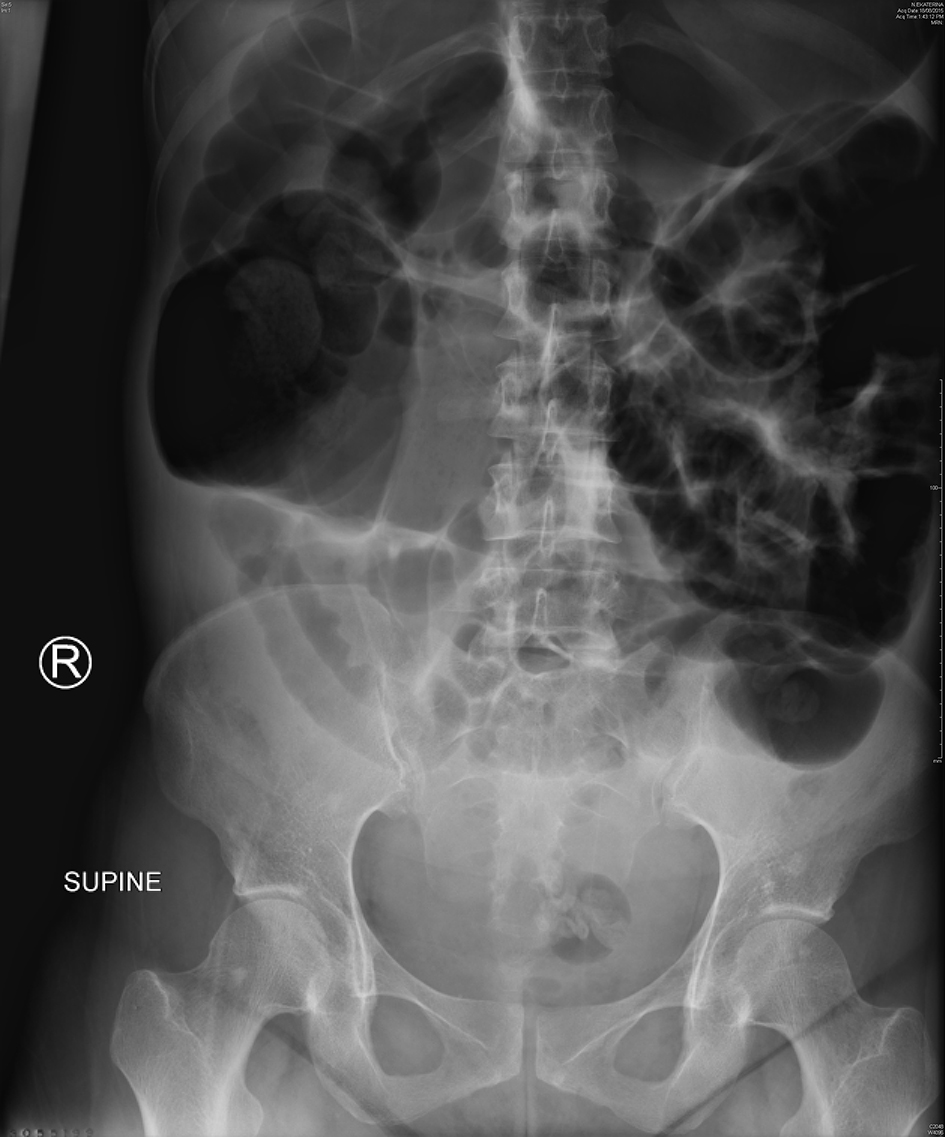 Click for large image | Figure 2. Abdominal X-ray of case 2 showing widespread gaseous distension of the small and large bowel. Cecal diameter measures 9.5 cm. |
Conservative management which comprised nasogastric tube insertion, intravenous fluids, analgesia and rectal laxative sodium phosphate was instituted. A rigid sigmoidoscopy was performed and a rectal tube was inserted after colonoscopic decompression. This provided symptomatic benefit with significantly reduced abdominal distension and bowel motions were subsequently passed the next day. She slowly returned to a normal diet and her recovery remained uneventful with discharge home on POD 4.
Case 3
A 40-year-old, para 3 woman had an emergency cesarean section at term after presenting in spontaneous labor prior to her scheduled repeat section. There were no operative complications or bowel involvement noted; however, she did suffer from significant nausea and vomiting and intravenous dexamethasone and droperidol were administered given her allergy to metoclopramide. On POD 2, she had intensifying abdominal pain and had not passed stool. Physical examination found a distended abdomen, generalized tenderness and audible bowel sounds. Vital signs and laboratory results were within normal range. Abdominal X-ray showed severe colonic dilation, no obvious air-fluid levels and no free gas under the diaphragm (Fig. 3). In discussion with the general surgeons, the plan was for conservative management with clear fluids, optimization of potassium and magnesium levels and rectal laxative sodium phosphate.
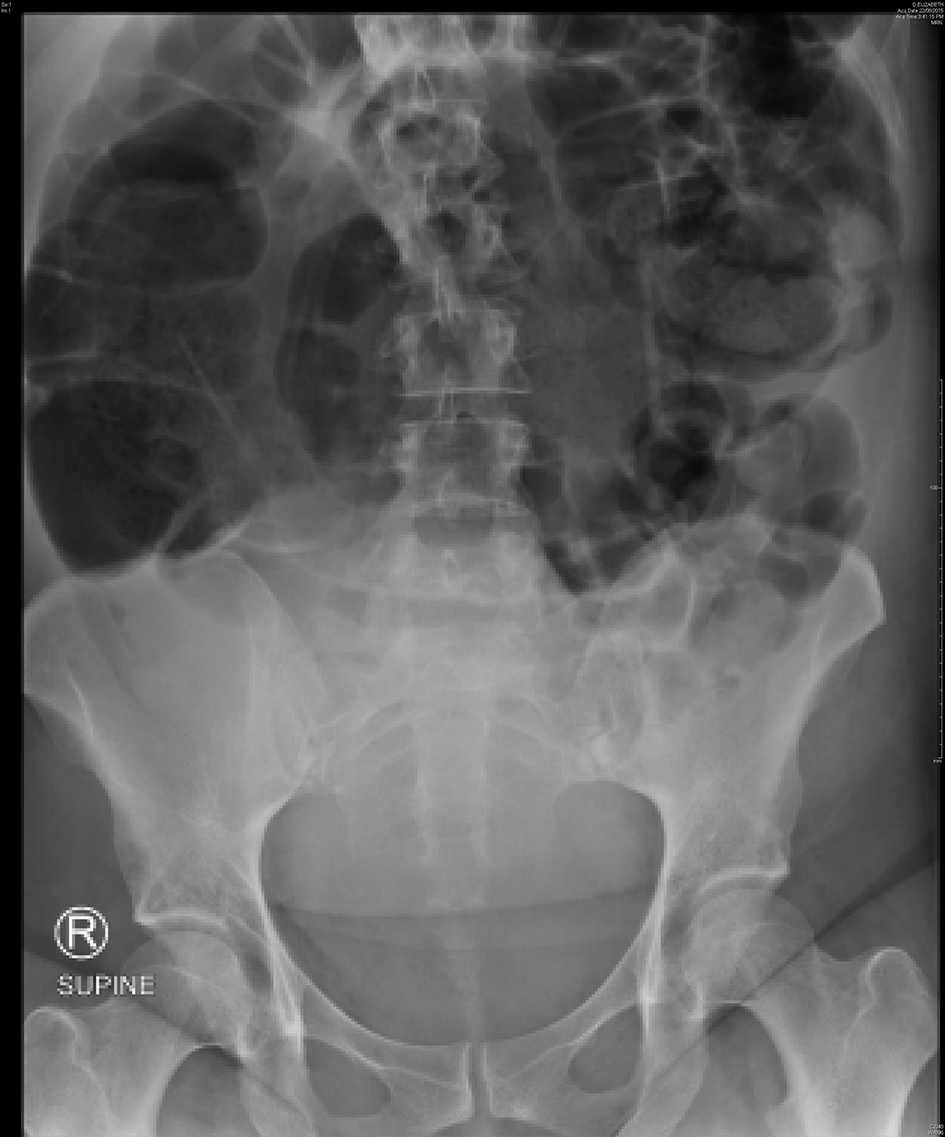 Click for large image | Figure 3. Abdominal X-ray of case 3 showing severe colonic dilation with the cecum measuring 15 cm. |
On POD 3, the patient became tachycardic. Surgical review occurred and CT with oral and intravenous contrast revealed a grossly distended cecum measuring 15 cm in diameter with transition point in the proximal descending colon. The diagnosis of an ACPO was made. Rigid sigmoidoscopy was attempted but unsuccessful. The patient was kept fasted and conservative management continued with non-opiate analgesia, regular oral and rectal laxatives and intravenous fluids. No improvement was noted. Intravenous neostigmine was administered prior to attempted colonoscopic decompression with rectal tube and a good response of flatus was seen. Repeat abdominal X-ray showed a reduced cecal diameter now measuring 8.5 cm. She reported mild improvement in both her abdominal pain and distension. However, on POD 4, she clinically deteriorated becoming febrile (temperature 37.9 °C) with a persisting sinus tachycardia. Her abdomen was diffusely tender to light palpation. Inflammatory markers continued to rise (CRP greater than 300 mg/L and neutrophils were 20 × 109/L) and there was a high level of suspicion of ischemic bowel. She proceeded to an emergency laparotomy where a significantly distended colon with cecal ischemia and perforation was found. A right hemi-colectomy was performed with primary anastomosis. Histology showed extensive transmural necrosis of the cecum with perforation, consistent with a diagnosis of ACPO. Postoperative recovery was unremarkable.
| Discussion | ▴Top |
Primary findings of the current report are that all post-cesarean patients developed a functional bowel obstruction. Two exhibited signs of imminent perforation from cecal dilation and required major surgical invention with a right hemi-colectomy. No additional contributing risk factors were identified from this case series.
The true incidence of OS is unknown as many mild cases resolve spontaneously and no reliable national or international data exist on its frequency [12]. In obstetrics, cesarean section with spinal anesthesia seems to be the most common operative procedure associated with this syndrome [13-17]; however, it has also been reported after cesarean hysterectomy [18] and vaginal births [15, 19]. There are no data on predisposing factors or any associated with respect to ethnic group, parity and indication for cesarean section which is also confirmed in this case series.
The pathogenesis of OS is not completely understood. Its association with trauma, spinal anesthesia and metabolic or pharmacological factors suggests impairment of the autonomic nervous system, leading to excessive parasympathetic suppression or sympathetic stimulation [20]. Further, it has been postulated that in the postpartum state, declining serum estrogen levels also causes a decrease in parasympathetic tone [21]. Interruption of the parasympathetic fibers from S2 to S4 leaves an atonic distal colon and a functional proximal obstruction [17, 22]. Subsequently, colonic diameter increases as does tension in its wall, thus increasing the risk of ischemia and perforation. The cecum, having the largest resting diameter to start, is the most easily dilated (Law of Laplace) and thus the most susceptible to ischemic necrosis and perforation [23]. A cecal caliber of 9 cm or greater is considered dilated [24]. The risk of colonic perforation rapidly increases when cecal diameter exceeds this [10, 11, 25, 26]. Retrospective data suggest a diameter equal to or greater than 12 cm is associated with an increased risk of cecal perforation and fecal peritonitis [10-18, 21, 27]. Therefore, cecal diameter appears to be a principal prognostic factor of morbidity and mortality associated with a functional bowel obstruction, regardless of the type, and subsequently should strongly dictate the approach to management.
In the early puerperium, clinically diagnosing colonic obstruction can be very difficult especially in cesarean patients with expected postoperative pain. A paralytic ileus refers to abdominal distension, intolerance of oral intake and an inability to pass flatus persisting for more than 3 - 5 days postoperatively [4, 28]. Examination typically reveals a hyper-resonant and distended abdomen, generalized tenderness and reduced to absent bowel sounds. In patients with OS progressive abdominal distention and associated abdominal pain occur in 90-100% and 80% of patients, respectively [12]. Nausea, vomiting and constipation are not consistently present. Clinical examination is similar to a paralytic ileus, except bowels sounds are higher pitched and hyperactive [1]. The presence of tachycardia, hypotension, pyrexia, localized right iliac fossa tenderness and the presence of peritoneal signs are suggestive of colonic ischemia or perforation [29]. Laboratory tests are generally non-diagnostic. A plain abdominal X-ray is the most useful diagnostic test to determine the extent of colonic dilation in patients with suspected functional bowel obstruction and can be obtained quickly in the acute setting. It can demonstrate a dilated cecum, often up to the splenic flexure and occasionally to the rectum. Thumb printing (the appearance of “thumb print” shaped projections from thickened haustra secondary to bowel wall edema) and pneumoperitoneum (the abnormal presence of air or other gas in the peritoneal cavity) are radiographic signs associated with bowel ischemia and perforation, respectively [30]. Abdominal CT is frequently required to confirm diagnosis of OS by revealing a transition point [31]. More often, the clinician is aware of the specific cecal diameter before a definitive diagnosis is made, thereby allowing for earlier decision-making based on this value and prompt initiation of appropriate management.
With regard to management, diagnosis of either an ileus or OS has been the main approach to patient care and often treatment has been delayed at this expense. Commonly, an ileus is managed with supportive care which involves removal of any precipitants (e.g. narcotic analgesia, anti-cholinergics), pain control that minimizes opioid use, correction of fluid and electrolyte imbalance, bowel rest, nasogastric suction and nutritional support. The management of OS has been previously classified into non-surgical and surgical, based on cecal diameter and/or the patient’s clinical state. This approach to care is therefore in support of our proposed argument that cecal diameter is a key prognostic factor rather than the specific diagnosis itself. Conservative management should be initiated in the first stance, when there is no pain and cecal distension is not extreme (i.e. less than or equal to 10 cm). Medical (e.g., neostigmine administration) or colonoscopic decompression is instituted if the patient does not improve after 1 - 2 days of conservative treatment or if the cecum is distended more than 9 cm in the absence of peritonitis or perforation [1, 32-34]. Surgery is carried out if pharmacologic or endoscopic attempts at bowel decompression fail or in an event of bowel ischemia or perforation [35]. With only less than 6% of operative mortality, surgery is a safe option especially given the fact that over 50% of the patients would otherwise die with necrotic or perforated bowel [10]. In the absence of bowel perforation or ischemia, cecostomy is the procedure of choice but if these complications have occurred, resection with or without primary anastomosis should be performed [18, 36].
Clinical deterioration of case 1 warranted abdominal exploration. A higher index of suspicion for cecal distension may have persuaded the obstetrician to seek an earlier plain abdominal X-ray for this symptomatic patient.
Early abdominal imaging was performed on case 3. Despite having a cecal diameter of 15 cm and being at higher risk of perforation, it was not unreasonable to try medical and endoscopic management, given that she showed no signs of complications at that time. The delay in surgical intervention was secondary to failure of medical management. Her clinical deterioration prompted an emergency laparotomy.
Appropriate management did ensue for case 2 who had widespread colonic dilation with no transition point. Colonic decompression and rectal tube insertion had good effect and her postoperative ileus resolved.
From review of literature, early diagnosis of OS is strongly emphasized as a factor that may reduce morbidity and mortality. Although we agree that a diagnosis is important, it should not delay management. Early recognition of cecal dilation by plain abdominal radiograph and prompt intervention when this diameter equals or exceeds 10 cm, with or without definitive diagnosis, is our recommended approach to the management of functional bowel obstructions in post-cesarean patients.
Conclusion
OS and paralytic ileus have similar presentations and similar clinical and therapeutic implications. Post-cesarean section patients who present with significant abdominal pain on POD 1 and/or signs and symptoms consistent with a bowel obstruction must be identified quickly and the obstetrician must keep paralytic ileus and OS high in the list of differential diagnosis. It can be difficult to establish the specific etiology for cecal dilation after cesarean section. However, ischemia and perforation remain the endpoint of progressive untreated distention and are feared complications of these two functional bowel obstructions.
Prompt measurement of cecal diameter with an abdominal X-ray is the most important aspect of care. Surgical review and intervention for those post-cesarean patients with a cecal diameter equal to or greater than 10 cm is essential.
Acknowledgement
The authors acknowledged Associate Professor Mark Umstad and Dr. Nicola Yuen for contributing to this article and for providing ongoing support.
Competing Interests
The authors declare they have no competing interests.
| References | ▴Top |
- Laskin MD, Tessler K, Kives S. Cecal perforation due to paralytic ileus following primary caesarean section. J Obstet Gynaecol Can. 2009;31(2):167-171.
doi - DePalma RT. Non-obstructive cecal dilation and perforation after caesarean section. Obstet Gynecol. 1978;52(1 Suppl):61S-63S.
pubmed - Jensen HK. Spontaneous perforation of the caecum following Caesarean section. Report of a case and review of the literature. Acta Obstet Gynecol Scand. 1972;51(4):381-383.
doi pubmed - Kalff JC, Wehner S, Litkouhi B. Postoperative ileus. In: UpToDate, Post TW (Ed), UpToDate, Waltkam, MA. (Accessed on October 14, 2015).
- Miedema BW, Johnson JO. Methods for decreasing postoperative gut dysmotility. Lancet Oncol. 2003;4(6):365-372.
doi - LaRosa JA, Saywell RM, Jr., Zollinger TW, Oser TL, Erner BK, McClain E. The incidence of adynamic ileus in postcesarean patients. Patient-controlled analgesia versus intramuscular analgesia. J Reprod Med. 1993;38(4):293-300.
pubmed - Saha AK, Newman E, Giles M, Horgan K. Ogilvie's syndrome with caecal perforation after Caesarean section: a case report. J Med Case Rep. 2009;3:177.
doi pubmed - Singh S, Nadgir A, Bryan RM. Post-cesarean section acute colonic pseudo-obstruction with spontaneous perforation. Int J Gynaecol Obstet. 2005;89(2):144-145.
doi pubmed - Tenofsky PL, Beamer L, Smith RS. Ogilvie syndrome as a postoperative complication. Arch Surg. 2000;135(6):682-686; discussion 686-687.
doi pubmed - Vanek VW, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie's syndrome). An analysis of 400 cases. Dis Colon Rectum. 1986;29(3):203-210.
doi pubmed - Eisen GM, Baron TH, Dominitz JA, Faigel DO, Goldstein JL, Johanson JF, Mallery JS, et al. Acute colonic pseudo-obstruction. Gastrointest Endosc. 2002;56(6):789-792.
doi - Carpenter S, Holmstrom B. Ogilvie Syndrome. 2014. [www.eMedicine.com/med/topic2699.htm].
- De M, Mandal A, Cooper JC. A case of Ogilvie's syndrome after caesarean section. J Obstet Gynaecol. 2002;22(6):686-687.
doi pubmed - Moore JG, Gladstone NS, Lucas GW, Ravry MJ, Ansari AH. Successful management of post-cesarean-section acute pseudoobstruction of the colon (Ogilvie's syndrome) with colonoscopic decompression. A case report. J Reprod Med. 1986;31(10):1001-1004.
pubmed - Nanni G, Garbini A, Luchetti P, Ronconi P, Castagneto M. Ogilvie's syndrome (acute colonic pseudo-obstruction): review of the literature (October 1948 to March 1980) and report of four additional cases. Dis Colon Rectum. 1982;25(2):157-166.
doi pubmed - Rodriguez-Ballesteros R, Torres-Bautista A, Torres-Valadez F, Ruiz-Moreno JA. Ogilvie's syndrome in the postcesarean section patient. Int J Gynaecol Obstet. 1989;28(2):185-187.
doi - Weber P, Heckel S, Hummel M, Dellenbach P. [Ogilvie's syndrome after cesarean section. Apropos of 3 cases. Review of the literature]. J Gynecol Obstet Biol Reprod (Paris). 1993;22(6):653-658.
- Hamed AD, Dare FO. Ogilvie's syndrome. Int J Gynaecol Obstet. 1992;37(1):47-50.
doi - Bhatti AB, Khan F, Ahmed A. Acute colonic pseudo-obstruction (ACPO) after normal vaginal delivery. J Pak Med Assoc. 2010;60(2):138-139.
pubmed - Rex DK. Acute colonic pseudo-obstruction (Ogilvie's syndrome). Gastroenterologist. 1994;2(3):233-238.
pubmed - Strecker JR, Jaluvka V. [Spontaneous cecum perforation following cesarean section]. Geburtshilfe Frauenheilkd. 1988;48(7):489-493.
doi pubmed - Ogilvie WH. William Heneage Ogilvie 1887-1971. Large-intestine colic due to sympathetic deprivation. A new clinical syndrome. Dis Colon Rectum. 1987;30(12):984-987.
doi pubmed - Jain A, Vargas HD. Advances and challenges in the management of acute colonic pseudo-obstruction (ogilvie syndrome). Clin Colon Rectal Surg. 2012;25(1):37-45.
doi pubmed - Herlinger H, Maglinte DDT. Small bowel obstruction. In: Herlinger H, Maglinte DDT, eds. Clinical radiology of the small intestine. Philadelphia: Saunders; 1989. p. 479-507.
- Camilleri M. Acute colonic pseudo-obstruction (Ogilvie's syndrome). In: UpToDate, Post TW (Ed), UpToDate, Waltkam, MA. (Accessed on October 14, 2015).
- Johnson CD, Rice RP, Kelvin FM, Foster WL, Williford ME. The radiologic evaluation of gross cecal distension: emphasis on cecal ileus. AJR Am J Roentgenol. 1985;145(6):1211-1217.
doi pubmed - Roberts CA. Ogilvie's syndrome after cesarean delivery. J Obstet Gynecol Neonatal Nurs. 2000;29(3):239-246.
doi pubmed - Townsend C, Beauchamp R, Evers B. Textbook of Surgery. The biological basis of modern surgical practice, 17th ed, Elsevier Saunders, 2004:110-113.
- Munro A. Large bowel obstruction. In: Wills BW, Paterson-Brown S, editors. Hamilton Bailey's Emergency Surgery. 13th ed. London: Hodder Arnold, 2000:436-439.
- Bullard KM, Dunn RD. Colon, rectum and anus. In: Brunicardi FCAD, Billiar TR, Dunn DL, Hunter JG, Matthews JB, Pollock RE, eds. Schwartz's Principles of Surgery. 9th ed. New York: Mc-Graw-Hill, 2010:1058-1059.
- Choi JS, Lim JS, Kim H, Choi JY, Kim MJ, Kim NK, Kim KW. Colonic pseudoobstruction: CT findings. AJR Am J Roentgenol. 2008;190(6):1521-1526.
doi pubmed - Harrison ME, Anderson MA, Appalaneni V, Banerjee S, Ben-Menachem T, Cash BD, Fanelli RD, et al. The role of endoscopy in the management of patients with known and suspected colonic obstruction and pseudo-obstruction. Gastrointest Endosc. 2010;71(4):669-679.
doi pubmed - Paran H, Silverberg D, Mayo A, Shwartz I, Neufeld D, Freund U. Treatment of acute colonic pseudo-obstruction with neostigmine. J Am Coll Surg. 2000;190(3):315-318.
doi - Reverdy D, Gebhart M, Kothonidis K, Gallez J, De Becker D, Liberale G. Pseudo-colonic obstruction after lumbar spine surgery: a case report. Acta Orthop Belg. 2006;72(6):769-771.
pubmed - Maloney N, Vargas HD. Acute intestinal pseudo-obstruction (Ogilvie's syndrome). Clin Colon Rectal Surg. 2005;18(2):96-101.
doi pubmed - Nadarajah R, Chin Tan JW, Tan YR, Lay KT. Ogilvie's syndrome with caecal perforation following caesarean section: A case report. J Med Cases. 2013;4(4):230-233.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
