| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 5, Number 4, December 2016, pages 106-111
Anesthesia With Propofol Does Not Reduce Interleukin-6 Release in Response to Abdominal Surgery of Varying Severity
Durk Fekkesa, c, Jaap W. Hola, b, Robert J. Stolkera
aDepartment of Anesthesiology, Erasmus University Medical Center, PO Box 2040, 3000 CA, Rotterdam, The Netherlands
bCurrent address: Department of Anesthesiology, Onze Lieve Vrouwe Gasthuis, PO Box 95500, 1090 HM, Amsterdam, The Netherlands
cCorresponding Author: Durk Fekkes, Department of Anesthesiology, Erasmus University Medical Center, Room H-1273, PO Box 2040, 3000 CA, Rotterdam, The Netherlands
Manuscript accepted for publication November 18, 2016
Short title: Propofol and IL-6 Response After Surgery
doi: https://doi.org/10.14740/jcgo413w
| Abstract | ▴Top |
Background: Data on whether anesthesia with propofol promotes or attenuates interleukin-6 (IL-6) production are conflicting. The purpose of this study was to investigate the effect of different doses of propofol, but similar doses of opiates, on plasma concentrations of IL-6 in patients undergoing two distinct operations of different severity and duration.
Methods: This study was a prospective, single-center, two-armed observational study. Blood samples were collected in 28 patients undergoing abdominal hysterectomy (H) or vulvectomy (V). IL-6 was measured 24 hours preoperatively, prior to induction, at the end of the operation, and at 24 and 96 hours postoperatively. We tested the effect of propofol on IL-6 levels with linear regression.
Results: IL-6 concentrations at the end of surgery were significantly higher in group H than in group V (38.6 (31.3) pg/mL vs. 3.7 (2.7) pg/mL, P < 0.001). Peak levels of IL-6 were seen in group H at the end of operation, while maximum levels in group V were attained 24 hours after surgery. No relationship between administered amount of propofol and IL-6 release could be identified. However, peak levels of IL-6 were significantly correlated to surgery duration in both group H and group V (r = 0.773, P = 0.002 vs. r = 0.568, P = 0.034).
Conclusions: Propofol does not diminish perioperative levels of IL-6 in patients undergoing abdominal hysterectomy or vulvectomy. The results also suggest that surgery duration is the main determinant of IL-6 response in both patient groups. Our findings indicate that there may be a clinical benefit to reduce the duration of surgery.
Keywords: Anesthetics IV; Propofol; Interleukin-6; Surgery duration
| Introduction | ▴Top |
There has been concern that anesthesia might play a role in causing pathological deactivation of the immune system, making the patient more susceptible to cancer metastasis, infection or sepsis after surgery [1, 2]. On the other hand, it has been thought that the potentially dampening effects of anesthesia could be useful to reduce the chance of a systemic inflammatory response syndrome from occurring during high risk surgery [3].
There is a great deal of information about the immunological impact of anesthetic drugs [4]. However, it is often unclear whether opiates or hypnotic drugs contribute to immunomodulation. There is evidence that opiates can influence the immune system, although results are inconsistent [5]. Other studies suggest that opioids have little effect on the secretion of the cytokine interleukin-6 (IL-6) [6-8]. IL-6 is an acute phase pro-inflammatory marker, which is an accepted early indicator of inflammation with peak plasma levels being proportional to the amount of surgical trauma [9, 10]. Hypnotic drugs have wide ranging effects on cytokines in vitro, but data are inconclusive [11-13]. Propofol has been found to inhibit IL-6 production by lipopolysaccharide-stimulated mononuclear cells in vitro [13]. In vivo data collected from rat studies revealed that propofol has anti-inflammatory properties and is able to dampen IL-6 increases [14]. A clinical study comparing anesthesia regimens found that an anesthetic using propofol and alfentanil reduced the release of IL-6 when compared to anesthesia maintained with isoflurane and nitrous oxide [2]. Gilliland et al [15] demonstrated that IL-6 response during abdominal surgery does not seem to be influenced by isoflurane and propofol. However, another clinical study suggested that propofol/fentanyl anesthesia promoted a pro-inflammatory state [16]. When considering these studies, it is clear that conflicting results are realized when propofol is used in different in vivo or in vitro experimental settings. Furthermore, most clinical studies were unable to isolate the individual effect of propofol from various confounders such as varying anesthesia regimens.
The aim of this study was to investigate whether the amount of propofol administered during surgery governs the response of IL-6. To this end, we determined plasma levels of IL-6 from patients who underwent two types of surgery of differing severity and duration (patients undergoing abdominal hysterectomy or vulvectomy), while receiving different amounts of propofol, but similar doses of the opiate sufentanil.
| Materials and Methods | ▴Top |
Study design
This study is a secondary analysis of a prospective and consecutive, single-center, two-armed observational study on the effects of anesthesia and surgery duration on the response of IL-6. The primary outcome for the current analyses was the effect of propofol on the postoperative release of IL-6 in plasma of 28 patients undergoing abdominal hysterectomy or vulvectomy. The secondary outcome was the effect of surgery duration on release of IL-6.
The protocol was approved by the Medical Ethics Committee of the Erasmus Medical Center, Rotterdam (MEC-2008-134). All procedures were performed in accordance with the Helsinki declaration. Informed consent was obtained from all subjects; the requirement of written informed consent was waived by the ethics committee. Inclusion criteria were: 1) scheduled for vulvectomy or abdominal hysterectomy, 2) expected surgery duration greater than 0.5 h, 3) age higher than 18 years, 4) American Society of Anesthesiologists (ASA) classification I-III, and 5) informed consent. Exclusion criteria were: 1) ASA-classification IV-V, 2) patients unable to speak Dutch, 3) and patients not able to consent. Patients had the right to withdraw from the study at any time. Patients who developed serious adverse side effects were to be withdrawn from the study. The subjects reported in the study have been previously reported [17, 18].
Patient management
All patients received a tablet of lorazepam 1.0 mg and of the selective COX-2 inhibitor celecoxib 100 mg approximately 1 h before surgery. Personal drug regimens were continued during the study. The observational nature of this study allowed the staff anesthesiologist to place an epidural catheter if the anesthesiologist felt it was indicated for adequate postoperative analgesia. Anesthesia was induced with propofol 1 - 2 mg/kg IV for sedation and sufentanil 0.15 - 0.30 mg/kg IV for analgesia. Cisatracurium provided muscle relaxation for optimal intubation conditions. Prior to the first incision, all patients received a combination of the antibiotics cefazoline 1 g and metronidazol 500 mg IV.
For all patients, the minimum postoperative pain control regimen included paracetamol 4,000 mg/24 h and celecoxib 200 mg/24 h. Morphine was titrated postoperatively until sufficient pain control was achieved (a visual analog scale (VAS) score of 4 or less). The daily regimen of paracetamol and celecoxib was continued until patients no longer experienced pain with VAS scores greater than 4. Patients with an epidural catheter had it removed when the anesthesiologist determined that it was no longer indicated for adequate pain control. Patient characteristics, medications used during and after surgery, and duration of surgery were documented.
Laboratory assessments
EDTA blood samples (4 mL) were collected in all 28 patients at 24 h preoperatively, right after IV canula placement prior to induction of anesthesia, at the end of the operation, and at 24 and 96 h postoperatively. Enzyme immunoassays for the quantitative determination of human IL-6 were performed with a sandwich ELISA (Pelikine CompactTM and additional Pelikine ToolsetTM, Sanquin, Amsterdam, The Netherlands) as described previously [17]. Data were calculated as pg/mL.
Statistical analysis
Data on patient characteristics and perioperative variables were analyzed using SPSS 16.01 for windows (SPSS Inc., Chicago, IL, USA). As the nature of this study was observational, we did not perform a power analysis. The independent sample t-test was used to compare means for patient demographics (excluding ASA classification) and perioperative characteristics. The Pearson Chi-square test was used to evaluate differences in ASA classification. The Fisher exact test was used to analyze differences in the type of pain control techniques used between groups (NSAID only, NSAID + opiates, NSAID + opiates + epidural). All continuous variables were presented as mean ± SD. Data on ASA classification were presented as counts.
The correlations between peak plasma IL-6 levels and all perioperative characteristics were assessed in both patient groups using the Spearman rank test or the Pearson’s correlation test in case of normal distributions. The Kolmogorov-Smirnov test was used for normal distribution. Calculations and regression lines were performed using GraphPad Software (version Prism 5, San Diego, USA). All data were reported as mean ± SD.
| Results | ▴Top |
Twenty-eight consecutive patients were included in the study; no patients were withdrawn from the study. The vulvectomy group (group V) contained 15 patients, while the abdominal hysterectomy group (group H) contained 13 patients. All patients were female and there was no significant difference in weight. Patients in group H were younger and had significantly lower ASA scores (Table 1). There was no significant difference in the amount of sufentanil used between groups. Group H had a significantly longer operating time (P = 0.002) and significantly more propofol was used in this group (P = 0.026) compared to group V. One of the patients received a blood transfusion with 285 mL of erythrocytes. Group H used significantly more crystalloids, colloids and more cisatracurium than group V (Table 1).
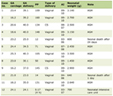 Click to view | Table 1. Characteristics and Perioperative Variables of the Subjects |
There was no significant difference in the amount of postoperative morphine, paracetamol or celecoxib given to both groups. Nine patients in the abdominal hysterectomy group were given preoperative epidural catheters for postoperative analgesia while only three patients were given one in the vulvectomy group. All epidural catheters were removed 24 h postoperatively, because pain control was found to be adequate.
Baseline levels of plasma IL-6 in group H (1.1 (0.8) pg/mL; 95% confidence interval (CI): 0.6 - 1.7) were not different from levels in group V (1.7 (0.8) pg/mL; CI: 1.2 - 2.2; P = 0.06). However, group H produced significantly more IL-6 than group V at the end of the operation (38.6 (31.3) pg/mL; CI: 19.8 - 57.4 versus 3.7 (2.7) pg/mL; CI: 1.4 - 6.1; P < 0.001). In addition, group H had peak levels of IL-6 at the end of the operation, while group V experienced peak levels (23.0 (20.1) pg/mL; CI: 11.9 - 34.2) 24 h after surgery.
There were no significant correlations between peak plasma levels of IL-6 and the amount propofol given for both group H (r = 0.522; CI: -0.52 to 0.84; P = 0.067; Fig. 1a) and group V (r = 0.029; CI: -0.50 to 0.55; P = 0.919; Fig. 1b). The results were found to be the same when the amount of propofol was designated as mg/kg body weight. On the other hand, the peak plasma levels of IL-6 were significantly correlated to surgery duration both in group H and in group V (r = 0.773; CI: 0.39 - 0.93; P = 0.002 and r = 0.547; CI: 0.05 - 0.83; P = 0.035; Figs. 2a and b, respectively). From Fig. 2, it can be seen that the slope of the regression line for group H was higher than for group V (0.519 versus 0.219). In both patient groups, there were no correlations between the plasma IL-6 levels after surgery on the one hand, and blood loss, colloids, crystalloids and cisatracurium given during surgery on the other hand. In addition, there was a positive correlation between the amount of propofol given and surgery duration in both group H (r = 0.521; CI: -0.04 to 0.83; P = 0.068) and group V (r = 0.454; CI: -0.09 to 0.79; P = 0.089), although no significance was reached.
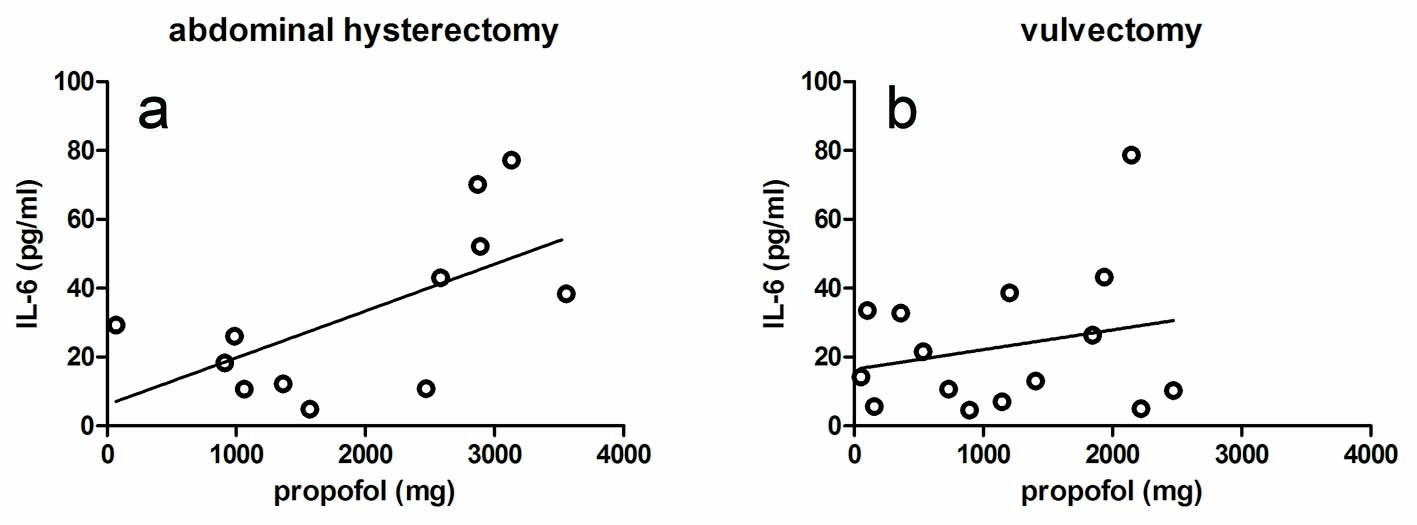 Click for large image | Figure 1. Effect of propofol on the peak levels of IL-6 in the plasma of the hysterectomy group (a) and the vulvectomy group (b). The correlations were not significant. |
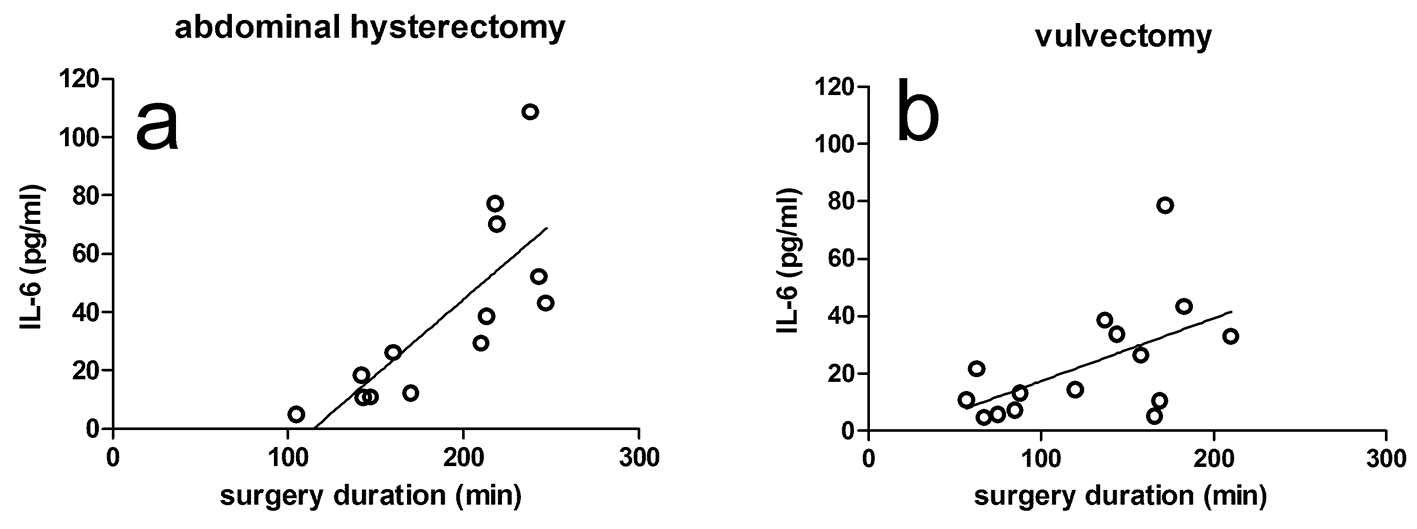 Click for large image | Figure 2. Effect of surgery duration on the peak levels of IL-6 in the plasma of the hysterectomy group (a) and the vulvectomy group (b). The correlations were significant in both groups. Surgery duration is defined as the time period between the start and the end of surgery. |
| Discussion | ▴Top |
The results of our study present evidence that there is no relationship between the amount of propofol administered during surgery and the perioperative response of IL-6. We found that plasma levels of IL-6 are highly correlated to surgery duration and that propofol does not seem to influence IL-6 response in patients undergoing abdominal hysterectomy or vulvectomy. One focus of this study was to isolate the effect of propofol from the potentially confounding effects of opiates. This was made possible by investigating how propofol influences IL-6 during two distinctive operations where sufentanil use was not significantly different. The experimental setup of this study is unique because it compares the relationship between IL-6 and two variables in two well-defined surgical settings of different severity. The two variables are the amount of propofol administered and duration of surgery. These comparisons were made during an abdominal surgical procedure expected to generate an intense IL-6 response, with another surgical procedure less prone to the same level of acute phase inflammatory reaction [9, 10].
The results of our study partly are in line with the results found by many other authors. Crozier and colleagues [2] concluded that anesthesia with alfentanil and propofol diminished the release of IL-6 in response to abdominal surgery when comparing to an anesthesia regimen using isoflurane. However, the authors suggested that this reduction was an effect of alfentanil and not propofol. Gilliland and colleagues [15] evaluated the effect of anesthesia with propofol or isoflurane on the balance of pro- and anti-inflammatory cytokine production during abdominal hysterectomy and they found similar levels of IL-6 in both groups. Kvarnstrom and colleagues [19] found no differences in IL-6 levels when comparing total intravenous anesthesia using propofol and inhalation anesthesia with sevoflurane and fentanyl. Two groups reported comparable results in patients undergoing other types of surgery. Baki and colleagues [20] found that the plasma concentrations of IL-6 during coronary artery bypass grafting were even higher in patients receiving propofol compared to desflurane anesthesia, while Margarit and colleagues [21] found no significant differences between the effects of total intravenous anesthesia with propofol and isoflurane anaesthesia on plasma levels of IL-6 after colorectal cancer surgery. Corcoran and colleagues [22] concluded that propofol may have attenuated the IL-6 response to cardiopulmonary bypass 4 h after reperfusion or that the higher IL-6 concentrations at 24 h merely represent a postponement of the inflammatory response.
However, our results are not in agreement with some other studies. Ke and colleagues [23] found that total intravenous anesthesia using propofol and remifentanil suppresses the inflammatory system caused during open cholecystectomy to a greater extent than isoflurane anesthesia. Takaono and colleagues [13] demonstrated that propofol inhibited IL-6 production by lipopolysaccharide-stimulated mononuclear cells from healthy volunteers, while Taniguchi and colleagues [14] found comparable results in an animal study. On the other hand, two in vitro studies with human lymphocytes [12, 16] demonstrated that anesthesia with propofol promotes proinflammatory immune responses. The differences found when comparing our data with those of previous studies may be explained by use of different study designs [23]. Furthermore, the other studies are animal studies [14] or in vitro studies [12, 13, 16].
Our results provide some interesting points. It is noteworthy that propofol was not immunosuppressive in the vulvectomy group, an operation expected to have less activation of the acute phase reaction. The amount of propofol used in the abdominal hysterectomy group, which is associated with the strongest acute phase reaction, even seemed to be positively correlated to IL-6, although no significance was reached (P = 0.067). However, our study showed that this positive correlation was probably due to the longer operating time of this group. In both patient groups, plasma levels of IL-6 were found to be correlated to surgery duration. Therefore, it may be concluded that propofol does not dampen or heighten plasma IL-6 levels, and that surgery duration or the type of surgery performed is the most important factor influencing IL-6 synthesis. This is also in agreement with a study by Cruickshank et al [9] who reported that the response of IL-6 correlated with the duration and severity of surgery.
Limitations of our study are the relatively small number of patients and that some of the patients had an epidural catheter for adequate postoperative analgesia. However, epidural analgesia is not expected to dampen the immune system. There is evidence that epidurals do not alter IL-6 profiles, thus it should not provide a confounding effect [2, 8, 24]. The ASA values in the vulvectomy group are higher than in the other group, which may be due to increased comorbidity (at higher age) in patients suffering from vulva carcinoma. However, this difference did not influence IL-6 levels at baseline. Operation time, blood loss, cisatracurium doses, colloids and crystalloids given were also higher in the hysterectomy group than in the vulvectomy group. However, there was no correlation between these parameters and IL-6 levels.
Our study is unique because we were able to determine the effect of different amounts of propofol on the release of IL-6 during two well-defined surgical settings of different severity in which the use of the opiate sufentanil was not significantly different. This experimental setup is different because it compares the relationship between plasma concentrations of IL-6 on the one hand and the amount of propofol administered and the length of the operation on the other hand.
Future studies are warranted with larger populations that measure the amount of anesthetic given, the duration or severity of surgery and the levels of the perioperatively released cytokines IL-1, IL-6 and tumor necrosis factor-α.
In conclusion, our findings suggest that propofol does not inhibit IL-6 release and that longer operating times are paired with increased IL-6 production. Our findings also emphasize that there may be a clinical benefit to reduce the duration of surgery as much as possible.
Funding
Institutional and department research budget.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Durk Fekkes helped conduct the study, analyze the data, and write the manuscript, reviewed the analysis of the data and approved the final manuscript. Jaap W. Hol helped design the study, conduct the study, analyze the data, and write the manuscript, has seen the original study data, reviewed the analysis of the data, and approved the final manuscript. Dr. Hol is the archival author. Robert J. Stolker helped design the study, analyze the data, and write the manuscript, has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
| References | ▴Top |
- Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105(2):106-115.
doi pubmed - Crozier TA, Muller JE, Quittkat D, Sydow M, Wuttke W, Kettler D. Effect of anaesthesia on the cytokine responses to abdominal surgery. Br J Anaesth. 1994;72(3):280-285.
doi pubmed - Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22(3):263-277.
doi pubmed - McBride WT, Armstrong MA, McBride SJ. Immunomodulation: an important concept in modern anaesthesia. Anaesthesia. 1996;51(5):465-473.
doi pubmed - Brix-Christensen V, Tonnesen E, Sorensen IJ, Bilfinger TV, Sanchez RG, Stefano GB. Effects of anaesthesia based on high versus low doses of opioids on the cytokine and acute-phase protein responses in patients undergoing cardiac surgery. Acta Anaesthesiol Scand. 1998;42(1):63-70.
doi pubmed - Taylor NM, Lacoumenta S, Hall GM. Fentanyl and the interleukin-6 response to surgery. Anaesthesia. 1997;52(2):112-115.
doi pubmed - Naito Y, Tamai S, Shingu K, Shindo K, Matsui T, Segawa H, Nakai Y, et al. Responses of plasma adrenocorticotropic hormone, cortisol, and cytokines during and after upper abdominal surgery. Anesthesiology. 1992;77(3):426-431.
doi pubmed - Moore CM, Desborough JP, Powell H, Burrin JM, Hall GM. Effects of extradural anaesthesia on interleukin-6 and acute phase response to surgery. Br J Anaesth. 1994;72(3):272-279.
doi pubmed - Cruickshank AM, Fraser WD, Burns HJ, Van Damme J, Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond). 1990;79(2):161-165.
doi - Bolke E, Jehle PM, Graf M, Baier A, Wiedeck H, Steinbach G, Storck M, et al. Inflammatory response during abdominal and thyroid surgery: a prospective clinical trial on mediator release. Shock. 2001;16(5):334-339.
doi pubmed - Salo M, Pirttikangas CO, Pulkki K. Effects of propofol emulsion and thiopentone on T helper cell type-1/type-2 balance in vitro. Anaesthesia. 1997;52(4):341-344.
doi pubmed - Rossano F, Tufano R, Cipollaro de L'Ero G, Servillo G, Baroni A, Tufano MA. Anesthetic agents induce human mononuclear leucocytes to release cytokines. Immunopharmacol Immunotoxicol. 1992;14(3):439-450.
doi pubmed - Takaono M, Yogosawa T, Okawa-Takatsuji M, Aotsuka S. Effects of intravenous anesthetics on interleukin (IL)-6 and IL-10 production by lipopolysaccharide-stimulated mononuclear cells from healthy volunteers. Acta Anaesthesiol Scand. 2002;46(2):176-179.
doi pubmed - Taniguchi T, Yamamoto K, Ohmoto N, Ohta K, Kobayashi T. Effects of propofol on hemodynamic and inflammatory responses to endotoxemia in rats. Crit Care Med. 2000;28(4):1101-1106.
doi pubmed - Gilliland HE, Armstrong MA, Carabine U, McMurray TJ. The choice of anesthetic maintenance technique influences the antiinflammatory cytokine response to abdominal surgery. Anesth Analg. 1997;85(6):1394-1398.
doi pubmed - Brand JM, Frohn C, Luhm J, Kirchner H, Schmucker P. Early alterations in the number of circulating lymphocyte subpopulations and enhanced proinflammatory immune response during opioid-based general anesthesia. Shock. 2003;20(3):213-217.
doi pubmed - Hol JW, Stolker RJ, Klimek M, Stronks DL, Fekkes D. The tryptophan kynurenine pathway, neopterin and IL-6 during vulvectomy and abdominal hysterectomy. J Biomed Sci. 2014;21:102.
doi pubmed - Hol JW, van Lier F, Valk M, Klimek M, Stolker RJ, Fekkes D. Effect of major and minor surgery on plasma levels of arginine, citrulline, nitric oxide metabolites, and ornithine in humans. Ann Surg. 2013;258(6):1072-1078.
doi pubmed - Kvarnstrom AL, Sarbinowski RT, Bengtson JP, Jacobsson LM, Bengtsson AL. Complement activation and interleukin response in major abdominal surgery. Scand J Immunol. 2012;75(5):510-516.
doi pubmed - Baki ED, Aldemir M, Kokulu S, Koca HB, Ela Y, Sivaci RG, Ozturk NK, et al. Comparison of the effects of desflurane and propofol anesthesia on the inflammatory response and s100beta protein during coronary artery bypass grafting. Inflammation. 2013;36(6):1327-1333.
doi pubmed - Margarit SC, Vasian HN, Balla E, Vesa S, Ionescu DC. The influence of total intravenous anaesthesia and isoflurane anaesthesia on plasma interleukin-6 and interleukin-10 concentrations after colorectal surgery for cancer: a randomised controlled trial. Eur J Anaesthesiol. 2014;31(12):678-684.
doi pubmed - Corcoran TB, Engel A, Sakamoto H, O'Shea A, O'Callaghan-Enright S, Shorten GD. The effects of propofol on neutrophil function, lipid peroxidation and inflammatory response during elective coronary artery bypass grafting in patients with impaired ventricular function. Br J Anaesth. 2006;97(6):825-831.
doi pubmed - Ke JJ, Zhan J, Feng XB, Wu Y, Rao Y, Wang YL. A comparison of the effect of total intravenous anaesthesia with propofol and remifentanil and inhalational anaesthesia with isoflurane on the release of pro- and anti-inflammatory cytokines in patients undergoing open cholecystectomy. Anaesth Intensive Care. 2008;36(1):74-78.
pubmed - Hole A, Unsgaard G, Breivik H. Monocyte functions are depressed during and after surgery under general anaesthesia but not under epidural anaesthesia. Acta Anaesthesiol Scand. 1982;26(4):301-307.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
