| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 7, Number 2, June 2018, pages 37-42
Incidence and Risk Factors of Uterine Scar Dehiscence Identified at Elective Repeat Cesarean Delivery: A Case-Control Study
Mohamad K. Ramadana, b, e, Samar Kassema, Saadeddine Itania, Loubna Sinnoc, Sara Husseinb, Rabih Chahinb, Dominique A. Badrd
aDepartment of Obstetrics and Gynecology, Makassed General Hospital, Beirut, Lebanon
bDepartment of Obstetrics and Gynecology, Rafik Hariri University Hospital, Beirut, Lebanon
cResearch Unit, Makassed General Hospital, Beirut, Lebanon
dDepartment of Obstetrics and Gynecology, Universite Libre de Bruxelles (FOSFOM), Belgium
eCorresponding Author: Mohamad K. Ramadan, Lebanese University, Makassed General Hospital, Beirut, Lebanon
Manuscript submitted February 12, 2018, accepted April 20, 2018
Short title: Incidence and Risk Factors of USD
doi: https://doi.org/10.14740/jcgo481w
| Abstract | ▴Top |
Background: The aim of this study was to determine the rate of uterine scar dehiscence among women undergoing elective repeat cesarean delivery and to investigate associated risk factors.
Methods: This was a retrospective case-control study of patients with previous one or more lower segment transverse cesarean incisions giving birth by elective repeat cesarean delivery at two tertiary-care centers over a period of 1 year. Demographic data, previous and current obstetric events were recorded and compared among women with or without uterine scar dehiscence.
Results: Among 588 patients included in this study, 27 cases of uterine scar dehiscence were identified with an incidence of 4.6%. This rate was not affected by maternal age, parity, co-morbidity, twin gestation, preoperative labor or previous preterm cesarean delivery. Factors significantly associated with uterine scar dehiscence were “preterm delivery” (OR: 2.76, 95% CI: 1.18 - 6.42), “tertiary cesarean delivery or higher” (OR: 2.56, 95% CI: 1.14 - 5.75) and “inter-delivery interval ≤ 24 months” (OR: 2.38, 95% CI: 1.04 - 5.44). No immediate adverse maternal or neonatal complications were noted.
Conclusions: Uterine scar dehiscence is not uncommon among women undergoing elective repeat cesarean delivery. Increased risk was associated with preterm delivery, tertiary cesarean delivery or higher and short inter-delivery interval of ≤ 24 months.
Keywords: Uterine scar dehiscence; Elective repeat cesarean deliveries; Risk factors; Preterm delivery; Inter-delivery interval
| Introduction | ▴Top |
Cesarean delivery (CD) is one of the most frequent surgical procedures that constituted the delivery method in up to 32% of births in USA in 2015 [1]. Eighty to ninety percent of women with previous CD are delivered by elective repeat CD (ERCD) in subsequent pregnancy [2, 3]. Multiple factors were responsible for declining trial of labor after cesarean (TOLAC) by most patients, rendering repeat CD the leading constituent of total CD [4]. Consequently, the number of women who will undergo multiple CD will eventually increase despite the increased risk of associated morbidities [5]. Previous CD is the most important risk factor for both uterine scar rupture (USR) and dehiscence (USD); hence, it is not surprising to witness a surge of both conditions that paralleled the recent increase in cesarean section rates [6].
Unlike USR, there is a paucity of studies dedicated to USD and the limited information was the byproduct of studies initially designed to study USR (mostly in patients undergoing TOLAC). This subsequently had led to more confusion and uncertainties among obstetricians with respect to its clinical significance. USD generally refers to an incomplete uterine scar disruption where the serosa remains intact and the fetus, placenta and umbilical cord remain contained within the uterine cavity [7]. Usually it is a clinically occult benign condition identified accidentally at ERCD.
This study was designed to explore the incidence of USD exclusively among women undergoing ERCD and to investigate the risk factors associated with this condition in our obstetric population.
| Methods | ▴Top |
This retrospective case-control study was executed in the period between March 1, 2014 and February 28, 2015 at Rafik Hariri University Hospital and Makassed General Hospital which are two tertiary care centers in Beirut, Lebanon. It was approved by the Institutional Review Board of both hospitals. Pertinent data were collected from patients’ electronic medical records at both hospitals; thus, informed consent was not needed. All women with previous lower segment transverse cesarean incisions undergoing ERCD were eligible for analysis. The case-group included women with USD diagnosed at the time of ERCD. The control-group included women without USD at the time of ERCD. Patients were excluded if they had previous classical cesarean incision, previous myomectomy, failed TOLAC, major fetal abnormalities or abnormally invasive placentae.
USD was considered only when diagnosed and recorded in the operative report directly by the surgeon. The precise definition of USD considered in the study was muscular disruption of any size with intact serosa. Thinning of the lower uterine segment of any degree was not considered as dehiscence. The terms used by some obstetricians as complete, incomplete or partial were also disregarded and only the presence or absence of dehiscence was considered in the analysis.
The studied variables included maternal characteristics (age, parity and smoking), obstetrical history (previous preterm birth, gestational age, order of CD, inter-delivery interval, twin gestation, presence or absence of labor at the time of CD (documented on routine pre-operative cardiotocogram), pain at the site of cesarean scar and technique of previous hysterotomy closure (single versus double layer closure). The presence of any medical co-morbidity was noted and scored according to the index suggested by Bateman et al [8]. Then, scores were grouped as “low-risk” (score ≤ 2) and “high-risk” (score > 2). Preterm delivery was defined as any delivery before 37 completed weeks of gestation as calculated according to an early pregnancy ultrasound. Patients with more than two previous CD were grouped under the category “tertiary CD or higher” and patients with inter-delivery interval of 24 months or less (period between last and current delivery) were grouped under the category “inter-delivery interval ≤ 24 months”.
Furthermore, short-term clinical follow-up (up till discharge from hospital) of women and newborns was obtained for each case and control. Maternal follow-up included need for transfusion, cesarean hysterectomy and mortality. Neonatal follow-up included birth weight, Apgar score at 5 min, Asphyxia Neonatorum, mortality and admission to neonatal intensive care unit (NICU). Asphyxia Neonatorum was defined as Apgar score of < 4 at 1 min and of < 7 at 5 min.
The data were analyzed using SPSS software, version 23.0 (IBM SPSS Statistics 23.lnk). Categorical variables were expressed as numbers (percentages) while continuous variables were presented as mean ± standard deviation. Bivariate analysis was carried out by using Chi-square for comparing categorical variables and Student’s t-test for continuous variables. A forward logistic regression model was constructed to study the weight of different factors that showed association on bivariate analysis. P value of < 0.05 was considered statistically significant.
| Results | ▴Top |
During the study period, 3,901 women were delivered at both institutions, of whom 2,473 delivered vaginally and 1,428 by cesarean. All women who had a history of myomectomy or classical incisions, underwent primary CD due to any cause or underwent secondary CD due to failed TOLAC, carried fetuses with major congenital anomalies, had abnormally invasive placenta or had incomplete data were excluded. A total of 588 patients were available for final analysis. Twenty-seven patients (4.6%) were diagnosed with USD and constituted the study group while the remaining 561 patients served as controls. The detailed sampling flowchart is presented in Figure 1. Women included in this study had a mean age of 28.9 ± 5.5 years and parity of 2.1 ± 1.5. Sixty patients (10.2%) had a Bateman morbidity index more than 2. Two hundred (34%) were smokers. Mean gestational age was 37.5 ± 1.8 weeks; 2.7% of cases were carrying twin gestation. The mean order of CD was 2.6 ± 0.8, and the mean inter-delivery interval was 36.5 ± 22.5 months. There was no statistically significant difference between the study and control groups concerning mean maternal age, mean parity, smoking, co-morbidities, twin gestation, previous preterm delivery and presence of labor at the time of CD. The gestational age in USD group was significantly lower than the control group (36.7 ± 1.8 and 37.6 ± 1.8 weeks respectively, P = 0.02). Preterm delivery was significantly higher in USD group (33.3% versus 15.2%, P = 0.012). Tertiary CD or higher was significantly associated with USD (63% versus 39%, P = 0.013). The inter-delivery interval ≤ 24 months was also associated with higher USD (66.7% versus 45.1%, P = 0.028) (Table 1).
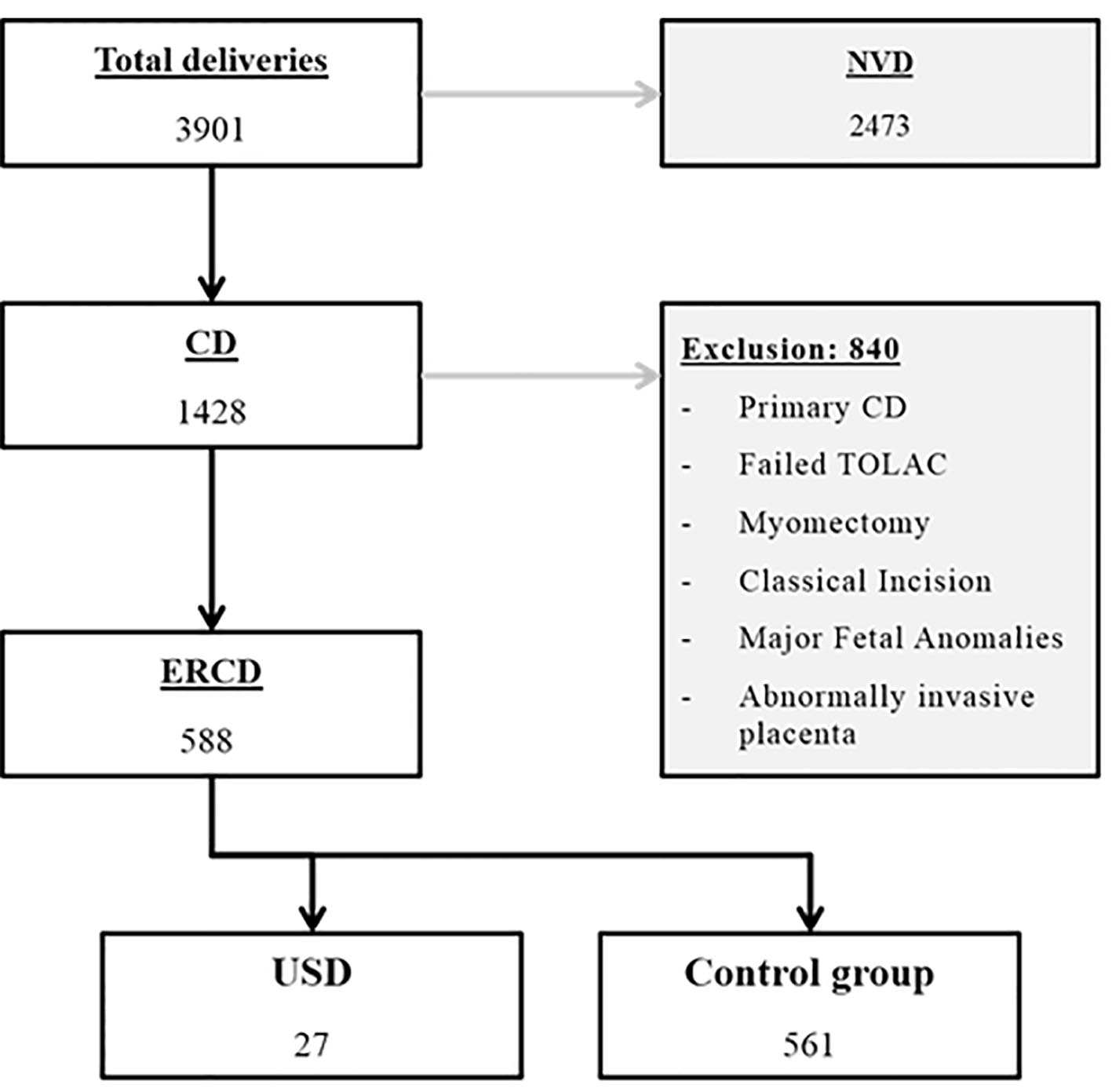 Click for large image | Figure 1. Flowchart of patients’ selection. CD: cesarean delivery; ERCD: elective repeat cesarean delivery; NVD: normal vaginal delivery; TOLAC: trial of labor after cesarean; USD: uterine scar dehiscence. |
 Click to view | Table 1. Comparison of Maternal Characteristics Between the Study Group and the Control Group |
Data about the pain at the site of cesarean scar and the technique of previous hysterotomy closure (single versus double layer closure) were available only from one hospital (Makassed General Hospital) and analysis showed no significant difference between the two groups. Forward logistic regression analysis was constructed to study the weight of risk factors (Table 2). It showed that “preterm delivery”, “tertiary CD or higher” and “inter-delivery interval ≤ 24 months” were significant and independent risk factors for development of USD with ORs and 95% CI of 2.76 (1.18 - 6.42), 2.56 (1.14 - 5.74) and 2.38 (1.04 - 5.44), respectively. Post-operative follow-up showed no difference in maternal hospital stay between the two groups (3.3 ± 3.6 days for USD group versus 2.5 ± 1 day for control group, P = 0.29) (Table 3). No blood transfusions, cesarean hysterectomies or ICU admissions were observed among women in either group. Newborns in the USD group had lower birth weight than those in the control group (2,812 ± 505 g versus 3,040 ± 504 g, P = 0.022) and tended to have higher rates of NICU admission (29.6% versus 13.2%, P = 0.016). However, Apgar score < 7 at 5 min and Asphyxia Neonatorum were not significantly different in both groups. Only one case of late neonatal death was recorded in the control group (Table 4).
 Click to view | Table 2. Comparison of the Pain at the Cesarean Scar and Mode of Previous Hysterotomy Closure Between the Study Group and the Control Group |
 Click to view | Table 3. Risk Factors for Uterine Scar Dehiscence |
 Click to view | Table 4. Comparison of Neonatal Outcomes Between the Study Group and the Control Group |
| Discussion | ▴Top |
USD complicated 4.6% of women undergoing ERCD in this study. There is wide variation in the reported incidence of USD in the literature, ranging from 0.3% to 19.4% [9]. This discrepancy is due to the inconsistency of a standardized definition for USD. Terms like scar disruptions, defects, windows, thinning of various degrees, symptomatic versus asymptomatic ruptures, complete versus incomplete or partial dehiscence were used. Even the collective term of rupture/dehiscence was erroneously employed to refer to this condition. Furthermore, incidence was occasionally determined among women delivered by CD in the immediate postpartum period or even some time later using imaging studies. The diagnostic tools were also variable and included vaginal exam following vaginal birth after cesarean, direct visualization at the time of CD after failed TOLAC, or at routine antenatal ultrasound exam (trans-abdominal or trans-vaginal) in subsequent pregnancies [7, 10-15].
The rate of preterm deliveries was significantly higher among the dehiscence group (33.3% versus 15.2%, P = 0.012). Other studies also reported similar finding. Bashiri et al found that preterm delivery is an independent risk factor of USD (OR: 1.79, 95% CI: 1.01 - 3.21, P = 0.048) [7]. They suggested an association between preterm delivery and uterine infection/inflammation that may have simultaneously led to weakness of the uterine scar.
Another important risk factor was ≥ 3 CD since patients in this category were more prone to develop USD (OR: 2.56, 95% CI: 1.14 - 5.74, P = 0.023). This conforms to the results reported by Bashiri et al who explained that this could be due to having thinner scars in those with higher number of CD [7]. This was also demonstrated by imaging of lower uterine segment among a group of women with previous CD. Using trans-vaginal ultrasound, Wang et al showed that women having more than one previous CD had larger scar defect (width and depth) compared to those with only one previous CD [9].
The third important risk factor was short inter-delivery interval of ≤ 24 months (OR: 2.38, 95% CI: 1.04 - 5.44, P = 0.04). Bujold et al reported that 10.5% of dehiscence occurred in patients with inter-delivery interval of less than 24 months compared to 3% after 24 months [16]. This finding may be related to the time needed for proper scar healing [17]. Using magnetic resonance imaging, Dicle et al showed that uterine scars needed at least 6 months to reach a normal appearance [18].
Unlike Bashiri et al who found that lower parity was associated with higher rates of dehiscence [7], parity in our study had no influence on uterine scar dehiscence. In spite of the general concept that twin pregnancy causes distension of the gravid uterus, we could not elicit any association of increased rate of dehiscence among pregnancies with twin gestations.
In this study, the presence of labor at the time of CD was noted to have no significant effect on the rate of dehiscence. This could be attributed to the fact that our patients were not allowed TOLAC and labor was experienced for short periods before proceeding directly to CD. In contrast, other studies which offered TOLAC showed that labor was a significant risk factor for USD [7, 19]. The absence of significant effect of labor on USD among patient undergoing ERCD might point to an event that preceded this pregnancy and probably started with defective healing of previous cesarean incision.
Roberge et al concluded in their study that the operative technique used for closure of the uterine scar, whether single or double layer, carried no significant effect on the risk of USD [20]. Same findings were confirmed in our study. Nevertheless, single layer closure of cesarean incision is a well-known risk factor of USR among women undergoing TOLAC [20].
Furthermore, we could not find an association between having pain at the cesarean scar and the risk for developing dehiscence. Similar observations were reported by Suzuki et al who concluded that there was no increased rate of dehiscence in patients who felt pain and tenderness in the lower uterine segment [21].
USD was not associated with maternal morbidity or mortality in the present study. However, it was significantly associated with NICU admissions and neonatal low birth weight. This could be explained by the fact that more premature infants were found to belong to USD group. Similar findings were reported by Bashiri et al [7].
Having a small sample size in the USD group was a limitation to this study. Another limitation was the inability to quantify the duration and intensity of labor which was mostly subjective.
This study was executed in two tertiary-care university teaching hospitals. Another aspect of strength was the paucity of publications with similar methodology, which makes this study, one of very few, addressing directly USD.
Conclusion
Factors associated with USD included “preterm delivery”, “tertiary CD or higher” and “short inter-delivery interval of ≤ 24 months”. The benign nature of this condition among our cohort is attributable to the fact that these are of low risk of developing USR. There is a great necessity to investigate the significance of USD among higher risk groups, as those planning for TOLAC.
Conflict of Interest
None.
| References | ▴Top |
- Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2015. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2017;66:1.
- Curtin SC, Gregory KD, Korst LM, Uddin SF. Maternal Morbidity for Vaginal and Cesarean Deliveries, According to Previous Cesarean History: New Data From the Birth Certificate, 2013. Natl Vital Stat Rep. 2015;64(4):1-13, back cover.
- Hamilton E, Platt R, Gauthier R, McNamara H, Miner L, Rothenberg S, Asselin G, et al. The effect of computer-assisted evaluation of labor on cesarean rates. J Healthc Qual. 2004;26(1):37-44.
doi pubmed - Guise JM, Denman MA, Emeis C, Marshall N, Walker M, Fu R, Janik R, et al. Vaginal birth after cesarean: new insights on maternal and neonatal outcomes. Obstet Gynecol. 2010;115(6):1267-1278.
doi pubmed - Silver RM, Landon MB, Rouse DJ, Leveno KJ, Spong CY, Thom EA, Moawad AH, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107(6):1226-1232.
doi pubmed - Sawada M, Matsuzaki S, Nakae R, Iwamiya T, Kakigano A, Kumasawa K, Ueda Y, et al. Treatment and repair of uterine scar dehiscence during cesarean section. Clin Case Rep. 2017;5(2):145-149.
doi pubmed - Bashiri A, Burstein E, Rosen S, Smolin A, Sheiner E, Mazor M. Clinical significance of uterine scar dehiscence in women with previous cesarean delivery: prevalence and independent risk factors. J Reprod Med. 2008;53(1):8-14.
pubmed - Bateman BT, Mhyre JM, Hernandez-Diaz S, Huybrechts KF, Fischer MA, Creanga AA, Callaghan WM, et al. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol. 2013;122(5):957-965.
doi pubmed - Wang CB, Chiu WW, Lee CY, Sun YL, Lin YH, Tseng CJ. Cesarean scar defect: correlation between Cesarean section number, defect size, clinical symptoms and uterine position. Ultrasound Obstet Gynecol. 2009;34(1):85-89.
doi pubmed - Thurmond AS, Harvey WJ, Smith SA. Cesarean section scar as a cause of abnormal vaginal bleeding: diagnosis by sonohysterography. J Ultrasound Med. 1999;18(1):13-16; quiz 17-18.
doi pubmed - Bromley B, Pitcher BL, Klapholz H, Lichter E, Benacerraf BR. Sonographic appearance of uterine scar dehiscence. Int J Gynaecol Obstet. 1995;51(1):53-56.
doi - Ofili-Yebovi D, Ben-Nagi J, Sawyer E, Yazbek J, Lee C, Gonzalez J, Jurkovic D. Deficient lower-segment Cesarean section scars: prevalence and risk factors. Ultrasound Obstet Gynecol. 2008;31(1):72-77.
doi pubmed - Baron J, Weintraub AY, Eshkoli T, Hershkovitz R, Sheiner E. The consequences of previous uterine scar dehiscence and cesarean delivery on subsequent births. Int J Gynaecol Obstet. 2014;126(2):120-122.
doi pubmed - Armstrong V, Hansen WF, Van Voorhis BJ, Syrop CH. Detection of cesarean scars by transvaginal ultrasound. Obstet Gynecol. 2003;101(1):61-65.
pubmed - Nielsen TF, Ljungblad U, Hagberg H. Rupture and dehiscence of cesarean section scar during pregnancy and delivery. Am J Obstet Gynecol. 1989;160(3):569-573.
doi - Bujold E, Mehta SH, Bujold C, Gauthier RJ. Interdelivery interval and uterine rupture. Am J Obstet Gynecol. 2002;187(5):1199-1202.
doi pubmed - Schwarz OH, Paddock R, Bortnick AR. The cesarean scar: An experimental study. Am J Obstet Gynecol. 1938;36:962-974.
doi - Dicle O, Kucukler C, Pirnar T, Erata Y, Posaci C. Magnetic resonance imaging evaluation of incision healing after cesarean sections. Eur Radiol. 1997;7(1):31-34.
doi pubmed - Landon MB, Hauth JC, Leveno KJ, Spong CY, Leindecker S, Varner MW, Moawad AH, et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351(25):2581-2589.
doi pubmed - Roberge S, Demers S, Berghella V, Chaillet N, Moore L, Bujold E. Impact of single- vs double-layer closure on adverse outcomes and uterine scar defect: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211(5):453-460.
doi pubmed - Suzuki S, Sawa R, Yoneyama Y, Asakura H, Araki T. Preoperative diagnosis of dehiscence of the lower uterine segment in patients with a single previous Caesarean section. Aust N Z J Obstet Gynaecol. 2000;40(4):402-404.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
