| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 9, Number 4, December 2020, pages 112-118
Successful Conservative Treatment of Ovulation-Related Hemoperitoneum in a Patient With Congenital Hypofibrinogenemia: A Case Report and Review of Literature
Mohamad K. Ramadana, b, c, Mariam Kharroubia, b, Janoub Khaza’ala, b, d
aMaternal-Fetal-Medicine Unit, Department of Obstetrics and Gynecology, Rafik Hariri University Hospital, Beirut, Lebanon
bDepartment of Obstetrics and Gynecology, Faculty of Medicine and Public Health, Lebanese University, Beirut, Lebanon
cMaternal-Fetal-Medicine Unit, Department of Obstetrics and Gynecology, Makassed General Hospital, Beirut, Lebanon
dCorresponding Author: Janoub Khaza’al, Maternal-Fetal-Medicine Unit, Department of Obstetrics and Gynecology, Rafik Hariri University Hospital, Beirut, Lebanon
Manuscript submitted March 2, 2020, accepted August 19, 2020, published online December 15, 2020
Short title: Hemoperitoneum and Hypofibrinogenemia
doi: https://doi.org/10.14740/jcgo628
| Abstract | ▴Top |
Intraperitoneal bleeding provoked by rupture of an ovarian follicle at ovulation or a corpus-luteum-cyst is not uncommon among women of reproductive-age. Usually, this is minimal and passes unnoticed. Massive hemoperitoneum is rare and is commonly associated with an underlying pathology. Patients with coagulation disorders or receiving anticoagulation therapy are exceptionally prone to develop massive hemoperitoneum that could at times be life-threatening if not identified and treated early. Very few cases of ovulation-related massive hemoperitoneum among afibrinogenemic/hypofibrinogenemic patients were reported to the literature. We present a case of congenital hypofibrinogenemia who presented with acute abdominal pain due to massive hemoperitoneum identified on ultrasonography. As the patient continued to be hemodynamically stable, on close monitoring of vital signs and serial hematologic parameters, conservative management with fresh frozen plasma (FFP) was started. This treatment modality was successful in inhibiting bleeding and ameliorating the clinical condition. The possibility of coagulation defects should be entertained in the differential diagnosis of acute abdomen with massive hemoperitoneum especially when no identifiable direct cause could be elicited. Conservative treatment aiming at conserving ovarian integrity and function has been successful in the management of cases with afibrinogenemic/hypofibrinogenemic and other bleeding disorders, thereby averting the need for surgical intervention. It should be the modality of choice in hemodynamically stable patients. Optimal management can be attained through careful clinical assessment and close follow-up by a multidisciplinary team including gynecologists, hematologists, surgeons and intensive care specialists. Ways to prevent recurrence should be accurately introduced to patients.
Keywords: Conservative treatment; Hypofibrinogenemia; Massive hemoperitoneum; Ruptured corpus luteum cyst; Ovulation
| Introduction | ▴Top |
At ovulation, rupture of the mature Graafian follicle occurs to release an oocyte. This process is usually associated with minute bleed and minimal pain [1]. This empty follicle (corpus luteum), in the absence of pregnancy, involutes to form the corpus albicans. Sometimes, blood or fluid accumulates inside the follicle, forming corpus luteum cyst. This highly vascular structure is prone to rupture which in healthy women is self-limited and clinically would pass unnoticed. On exceptional occasions, ovulation-related hemoperitoneum can cause acute abdomen with possible circulatory collapse necessitating surgical intervention [2]. If not recognized and treated early, it can lead to serious consequences including hemorrhagic shock and even mortality [3]. This is extremely rare in healthy women. Women with bleeding disorders or receiving anticoagulant therapy are more prone to develop this complication [4]. Congenital hypofibrinogenemia is a hereditary autosomal recessive hematologic disorder characterized by insufficient fibrinogen level (coagulation factor 1) [5]. Unlike afibrinogenemia, most patients with this condition are asymptomatic with only few cases diagnosed during infancy [5]. Reports of ovulation-related hemoperitoneum in women with congenital fibrinogen deficiency are scarce and most patients required surgical intervention [1, 6-12]. Herein, we report a case of 26-year-old woman, known to have congenital hypofibrinogenemia, presented with acute abdomen due to spontaneous massive hemoperitoneum presumably related to ovulation. She was successfully treated with fresh frozen plasma. Three weeks later no intraperitoneal collection could be identified. We then present a review of similar case reports published to the English literature.
| Case Report | ▴Top |
A 26-year-old nulliparous woman presented to the emergency department (ED) complaining of severe pelvic-abdominal pain of 1-day duration. She described the pain as cramps radiating to her back and shoulders associated with nausea and dysuria. She claimed to have had missed her period for 2 days, but rapid urine pregnancy test was negative. The patient reported being diagnosed with hypofibrinogenemia at age 9, after experiencing intractable bleeding following dental extraction. Her fibrinogen level was then < 100 mg/L. Since then, she reported no encounter of any abnormal bruising or bleeding. Her menarche was at age of 12, with regular menses without experiencing any abnormal uterine bleeding. At presentation, the patient looked oriented but with discomfort and apprehension. No respiratory distress, tachycardia, hypotension or other signs of circulatory collapse. The abdomen was mildly distended, soft with mild bilateral lower quadrants tenderness. Laboratory studies showed white blood cells (WBCs) of 10,000/µL, hemoglobin (Hb) of 11 g/dL, hematocrit (Hct) of 32.2% and platelets count of 216,000/µL. Serum β-human chorionic gonadotropin (hCG) and urine analysis were negative as were the liver function tests (LFTs). Coagulation profile showed prothrombin time (PT) of > 120 s, activated partial thromboplastin time (aPTT) > 120 s, international normalized ratio (INR) > 7 and fibrinogen of 0.64 mg/dL. Ultrasound disclosed diffuse hemoperitoneum with two left ovarian cysts measuring 4 × 3.3 and 4 × 3.4 cm, thin walled, smooth borders, unilocular with uniform hypoechogenic fluid contents highly suggestive of simple ovarian cysts (Fig. 1). After ruling out other possible causes of hemoperitoneum the patient was started on fresh frozen plasma (FFP) transfusion for a total of 6 units within 48 h after consulting with a hematologist. Twelve h later, hemoglobin dropped from 11 to 7.6 g/dL, for which 2 units of packed red blood cells (pRBCs) were transfused. Intravenous (IV) amoxicillin-clavulanate was started to prevent secondary infection. Repeat laboratory workup showed amelioration of her coagulation profile and stabilization of hemoglobin level. Transvaginal ultrasound (TVUS) imaging was done every other day showing steady regression of the hemoperitoneum volume in spite of a mild initial increase in the volume of one of the cysts. The patient continued to be clinically stable but was kept under close surveillance at hospital for 1 week. Coagulation profile was corrected within 48 h of admission (Fig. 2). She received a total of 10 units of FFPs during hospitalization.
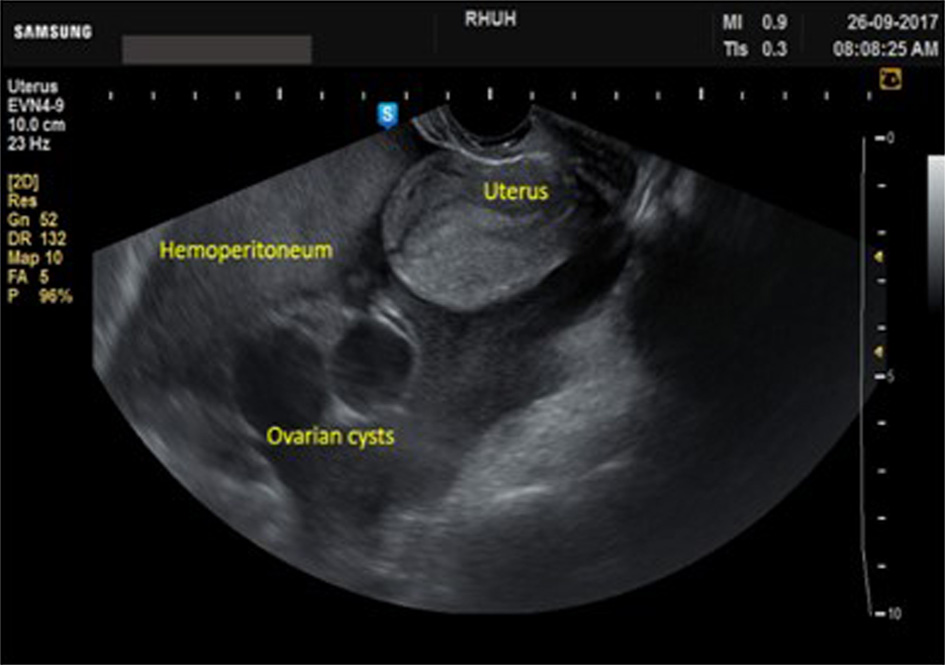 Click for large image | Figure 1. Transvaginal ultrasound at admission showing the massive hemoperitoneum and the two ovarian cysts. |
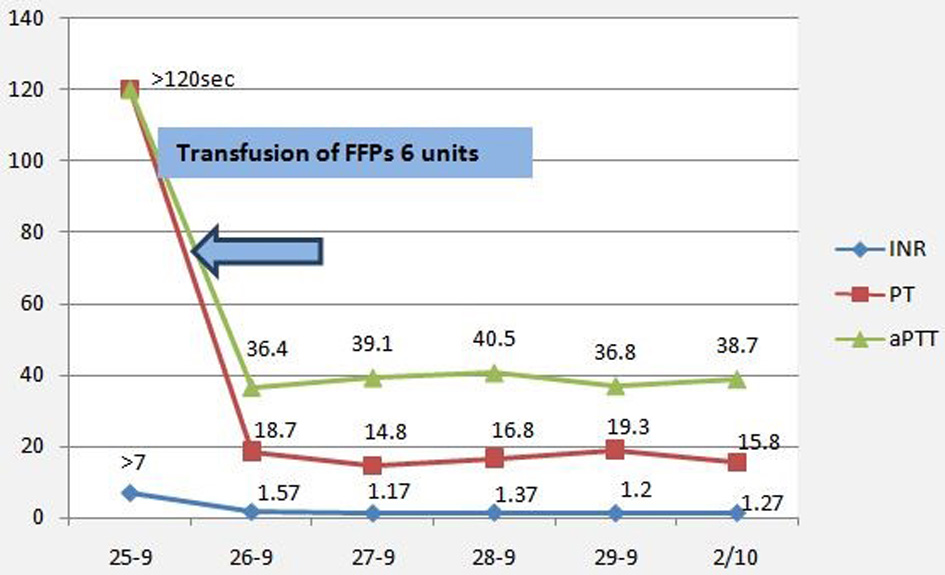 Click for large image | Figure 2. Timetable of coagulation profile. FFP: fresh frozen plasma; INR: international normalized ratio; PT: prothrombin time; aPTT: activated partial thromboplastin time. |
Two weeks after discharge, ultrasound showed complete resolution of the hemoperitoneum with a significant decrease in both ovarian cysts (Fig. 3). She was counseled and accepted starting on continuous low-dose oral contraceptive pills (OCPs) to prevent subsequent ovulation-related bleeding episodes. Unfortunately, we do not have long-term clinical follow up on this patient.
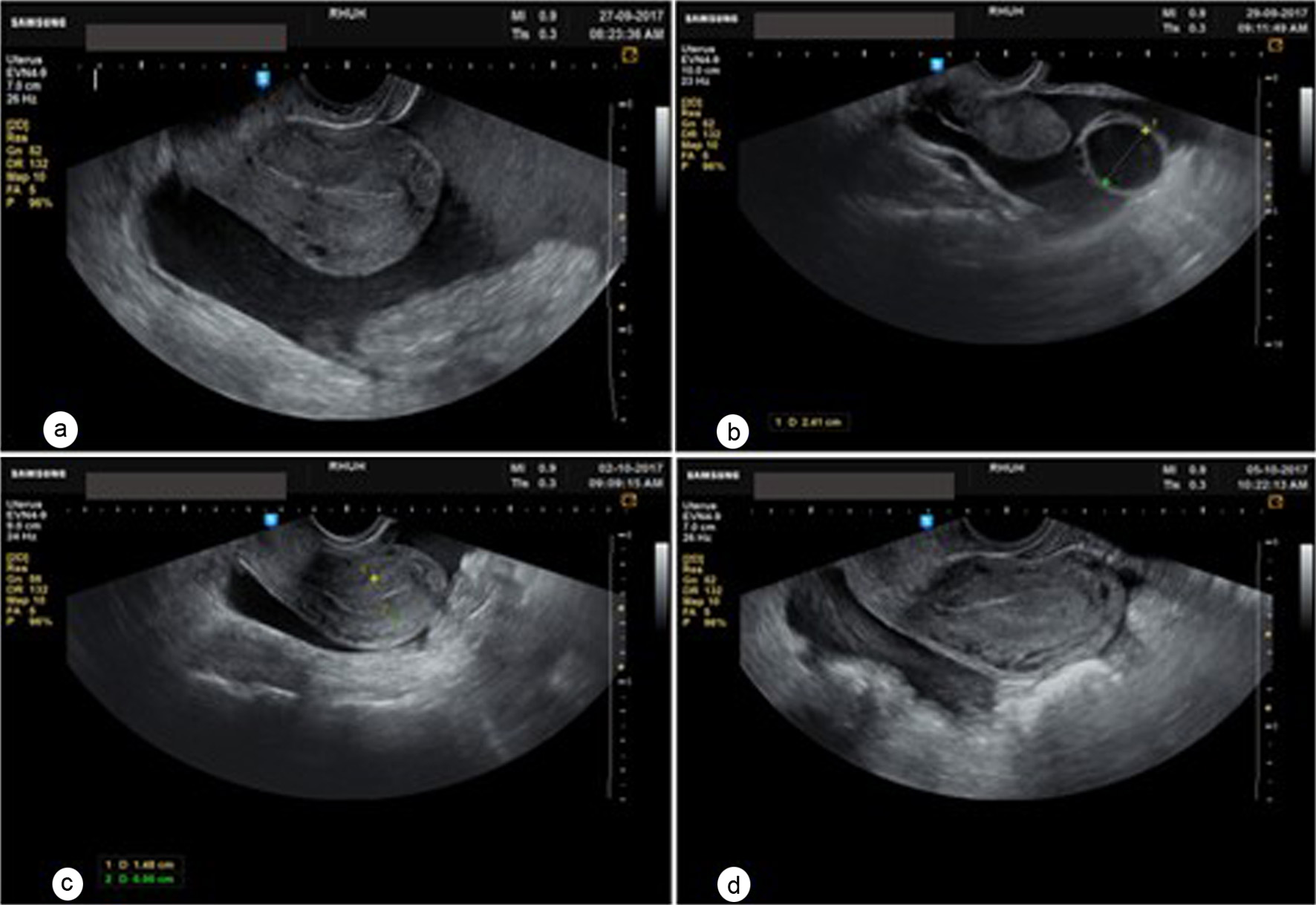 Click for large image | Figure 3. Transvaginal ultrasound showing the progression of the hemoperitoneum. (a) On day 2, (b) day 4, (c) day 6 of admission. (d) 4 days after discharge. |
| Discussion | ▴Top |
Fibrinogen is a glycoprotein synthesized by hepatocytes, with normal circulating levels ranging between 200 - 400 mg/dL and a half-life of 4 days [13]. It has a crucial role in maintaining hemostasis, by acting as a substrate for thrombin in the formation of insoluble fibrin clot [14]. Fibrinogen aids platelets by binding to glycoprotein IIb/IIIa on the surface of activated thrombocytes. The fibrin clot activates the system of fibrinolysis. Afibrinogenemia is a condition when no fibrinogen is detected while hypofibrinogenemia is a less serious condition when fibrinogen is < 150 mg/dL [5]. It is an autosomal recessive disorder affecting males and females equally, and more frequent in consanguineous marriages without ethnic predilection [15]. The prevalence of afibrinogenemia is estimated at 1 - 2/million [5]. The actual incidence of hypofibrinogenemia is really unknown as many individuals carrying this abnormality are asymptomatic [14]. Because hypofibrinogenemia with fibrinogen levels below 150 mg/dL is often caused by heterozygosity for a fibrinogen gene mutation, it is much more frequent than afibrinogenemia [14]. Clinical manifestations vary from being asymptomatic to developing catastrophic life-threatening bleeds. Afibrinogenemia has been reported to be associated with omphalorrhagia soon after birth in 85% [16]. Spontaneous splenic rupture is more frequent among patients with congenital fibrinogen deficiency, compared to other coagulation disorders and is considered to be specific to congenital fibrinogen deficiency [15, 17]. Intracranial hemorrhage, hemarthrosis, ovulation-related hemoperitoneum and delayed wound healing were reported among these patients. The most unorthodox complication is the paradoxical occurrence of thrombotic events following replacement therapy or occurring spontaneously [18]. There is an increased frequency of gynecologic and obstetric hemorrhage, such as menorrhagia, menometrorrhagia, spontaneous recurrent abortions, in addition to antepartum and postpartum hemorrhage in these patients [5]. The severity and frequency of bleeding episodes differ among individuals [1]. Unlike other coagulopathies, afibrinogenemia requires little maintenance therapy [6].
Hypofibrinogenemia has been clinically linked to wide range of hemorrhagic complications starting with asymptomatic minor bleed to more significant bleeding. It is associated with fewer bleeding episodes as compared to afibrinogenemia and may not be diagnosed until a traumatic or surgical challenge occurs [5]. Spontaneous bleeding in hypofibrinogenemic patients correlates with the level of serum fibrinogen. In the study of Peyvandi et al on 100 cases with fibrinogen deficiency, they found that in patients with fibrinogen levels > 10 mg/dL, hemorrhages were less frequent than in patients with severe deficiency. There was no case of central nervous system (CNS) and intra-retroperitoneal bleeding, thrombosis and miscarriage [15]. In fibrinogen deficiency, most fibrin-based coagulation tests (PT, aPTT) are invariably prolonged, but the most specific test being prolonged is thrombin time (TT) [5]. Usually fibrinogen level in most of the asymptomatic patients is around 100 mg/dL enough to prevent spontaneous bleed; however, they become symptomatic when subjected to trauma or surgery [14]. The diagnosis in this case was based on high clinical suspicion, laboratory data, and ultrasound findings. Despite that the underlying disease was disclosed to us by the patient, yet the source of bleeding had to be determined. The challenge was to figure the gravity of the situation and whether it was an ongoing bleed. The abdomen was not tense, and the patient was not in respiratory distress, neither had she any sign pointing to any organ dysfunction commonly seen in abdominal compartment syndrome. Spontaneous splenic rupture, which has been reported with this disorder, was less likely as it usually presents with left upper quadrant pain with radiation to the left shoulder. On the other hand, the timing of hemoperitoneum (luteal phase) together with stability of the patient encouraged us to initiate immediately the conservative medical therapy. Even with the remote possibility of splenic rupture, this management modality has been successful in the treatment of splenic rupture [17]. The need for surgical exploration and intervention was postponed pending any deterioration in the hemodynamic status of the patient who was kept under close continuous surveillance of vital signs, serial Hb measurements and regular ultrasound assessment of hemoperitoneum. It was also important to follow the stability of the patient and the response to this modality as this treatment has been previously reported to fail in stopping bleeding, where surgical intervention and resection of ovarian tissues was done in order control bleeding [6-8].
Ovulation-related hemoperitoneum is rare in normal women but has been described in patients with coagulopathies. This has been reported to complicate patients receiving anticoagulation, as well as those with several forms of bleeding disorders such as hemophilia A and B, fibrinogen deficiency, factor X, XIII, V, II and VII deficiencies, Von Willebrand disease (VWD), thrombocytopenia as with immune thrombocytopenic purpura (ITP) and pancytopenia as with aplastic anemia [18-21]. Treatment of fibrinogen deficiency is targeted at preventing and treating bleeding, thrombosis as well as preventing any obstetrical complication, together with preserving the ovarian function. For patients with coagulation disorders, the management decision should be based on the initial hemodynamic status of the patient, the improvement, stability or deterioration of the condition rather than the initial volume of hemoperitoneum. In favorable conditions, conservative therapy with replacement of fibrinogen should be immediately instituted with close monitoring of vital signs, serial sonographic estimation of hemoperitoneum volume and serial determination of Hct concentration. If improvement and stabilization continue, conservative management should be maintained till adequate level of fibrinogen is attained (> 150 mg/dL). If hemodynamic status deteriorates, surgical intervention might not be avoidable. When surgical intervention is deemed necessary, laparoscopy has been reported to successfully achieve hemostasis of the bleeding corpus luteum without the need to remove the entire ovary [22]. The presence of large volume hematoma should not preclude the use of laparoscopy. Evacuation of the peritoneal blood can be done with the use of 10 mm entry-port and a large sized irrigation-suction tool after lavage of the peritoneal cavity with 2 - 3 L of sterile water. Coagulation of corpus luteum bleeding edge can be achieved with bipolar diathermy. In a study of healthy 91 women with ruptured corpus luteum cyst (RCLC) and hemoperitoneum, most cases (81.3%) required laparoscopic intervention to achieve hemostasis [23]. The relatively higher success of conservative medical treatment in patients with coagulopathies could be related to the propensity of tiny vessels at the surface of the ovary responsible for the bleed. These vessels are more amenable to occlude once the body regains the ability to form fibrin plugs following replacement therapy. Unlike hemoperitoneum triggered by ruptured ectopic pregnancy or traumatic ruptured liver or spleen hematomas where the severed vessels are probably larger and less responsive to occlusion by mechanisms such as vasoconstriction and fibrin clot occlusion.
In contradistinction to other disorders of coagulation, patients with afibrinogenemia/hypofibrinogenemia are inclined to develop thrombotic events. This complication can involve venous, arterial or both systems and rarely can cause fatal pulmonary embolism [24]. Although the risk is increased following surgery, postpartum or following replacement with fibrinogen, yet in 50% of cases, thrombosis was reported to occur spontaneously [25].
The fundamentals of conservative treatment are fibrinogen supplementation. Fibrinogen concentrate is the treatment of choice. If not available, the virally inactivated FFP or cryoprecipitate is recommended. Virally-inactivated-FFP is preferred to cryoprecipitate when volume overload is not an issue. FFP which contains factors VIII, VWD and fibrinogen is of benefit in the initial bleeding episode [7].
In reproductive-age females with congenital fibrinogen deficiency or other coagulation disorders, presenting with spontaneous ovulation-related hemoperitoneum, oral contraceptives are helpful to suppress ovulation, thereby preventing recurrence of acute bleeding episodes [8, 9]. It can be used cyclically with 1-week interruption or used continuously if menorrhagia is an added problem. [26] The use of regular replacement therapy is controversial. The choice of putting patients on chronic replacement therapy must be individualized and management should on a case-per-case basis. The evidence is that spontaneous bleeding among patients with fibrinogen deficiency is uncommon. The mean annual incidence of bleeding episodes was 0.5 for patients on prophylactic replacement therapy and 0.7 for patients on on-demand therapy [15]. When considering assigning afibrinogenemic patients to chronic replacement therapy, physicians should acknowledge that the use of fibrinogen concentrate can transmit viral infections as hepatitis or human immunodeficiency virus (HIV), potentiate the risks for thrombosis and develop antibodies which limit its use for future episodes [5]. Regular replacement might be appropriate for those with frequent bleeding episodes and is recommended during pregnancy [27].
In spite of a recent trending towards conservative medical treatment, a standardized management is not defined in relatively stable cases [28]. We performed a search in the Medline database for articles published in English literature. We used the following terms: “afibrinogenemia” or “hypofibrinogenemia” and “hemoperitoneum” and “ovulation” or “rupture corpus luteum” to retrieve three articles. A similar search was then performed in Google Scholar using the same terms to retrieve 11 articles. Reference lists of retrieved articles were used to find more related publications. A total of eight relevant case reports were deemed appropriate and were used in our review. Only one case had hypofibrinogenemia [12] while the remaining 7 cases had afibrinogenemia [1, 6-11]. Two cases gave a history of hemoperitoneum whereas the remaining five described the experience with current patients presenting with hemoperitoneum. The relevant clinical findings of these case reports including treatment modalities were displayed in Table 1 [1, 6-12]. The paucity of reported cases suffering from serious gynecological bleeding associated with this condition compared to other bleeding disorders was remarkable. Two of these reports were concerned with promoting the prophylactic use of OCPs in preventing recurrence in patients with afibrinogenemia and a history of hemoperitoneum. Castaman et al reported the benefit of OCPs in preventing recurrence of ovulation-related hemoperitoneum. His patient had previously suffered two episodes when she was treated with wedge resection of both ovaries on two occasions soon after menarche. After the use of OCPs, she suffered no more recurrence [8]. A similar case with one previous episode of ovulation-related hemoperitoneum was reported by Henselmans et al. Similarly, the use of OCPs prevented recurrence [9]. Despite that the first case of afibrinogenemia was described in 1920 [29] and that of hypofibrinogenemia was reported in 1935 [30], yet the first case of ovulation-related hemoperitoneum in connection with congenital afibrinogenemia was reported 60 years later in 1981 [6]. Bleeding in congenital fibrinogen deficiency can be severe and occasionally fatal. These patients often have a milder disease than hemophiliacs and may be free of bleeding for long periods of time [28]. Some reviews stated that surgical intervention was used in 80% of patients with hemoperitoneum due to RCLC, others claimed that conservative medical replacement therapy was satisfactory in stopping bleeding in 80% of their patients [2, 31]. These reviews were actually on healthy females presenting with hemoperitoneum following RCLC; hence extrapolation of evidence to patients with bleeding disorders might not be appropriate. We could not elicit any change in the trend of treatment modality and could not confirm the observations made by Acharya et al who had previously stated that surgical intervention was common in early reports while newer reports resorted to medical replacement with the deficient factor for treatment [5]. Surgical intervention with concomitant oophorectomy, wedge resection or coagulation of the bleeding point were used in older reports in this review, nevertheless, this was also described in recent reports where surgery was performed after failure of conservative medical therapy to halt bleeding in two cases [11, 12].
 Click to view | Table 1. Cases of Hemoperitoneum due to Ovulation or Corpus Luteal Rupture in Patients With Fibrinogen Deficiency |
In conclusion, ovulation-related hemoperitoneum is rare, yet is a potentially life-threatening condition especially in patients with coagulation disorders. Gynecologists should recognize the potential of such complication in all females with congenital fibrinogen deficiency disorders after menarche. Optimal management is through careful clinical initial assessment by a multidisciplinary team including gynecologists, hematologists and intensive care specialists. This also implies close surveillance for the disease and treatment-related complications in a comprehensive care setting. Surgery may be avoided under circumstances of close clinical monitoring. Ways to prevent recurrence should be accurately implemented including routine vaccination against hepatitis, inhibiting ovulation and appropriate prophylactic replacement before surgeries and dental procedures or during pregnancy.
Acknowledgments
We would like to thank Ms. Loubna Sinno, the Research Coordinator at Makassed General Hospital, for assisting in the literature review.
Financial Disclosure
The authors have no support or funding to report.
Conflict of Interest
The authors have declared that no competing interest exists.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
MK Ramadan wrote the discussion and preformed the literature review. M Kharroubi wrote the introduction and case description. J Khaza’al wrote the introduction and case description. All authors read and approved the final paper.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
aPTT: activated partial thromboplastin time; AF: atrial fibrillation; APLS: anti-phospholipid syndrome; WBC: white blood cell; CL: corpus luteum; ED: emergency department; FFP: fresh frozen plasma; GI: gastrointestinal; Hb: hemoglobin; Hct: hematocrit; LFTs: liver function tests; MHVR: mechanical heart valve replacement; OCPs: oral contraceptive pills; PT: prothrombin time; RCLC: ruptured corpus luteum cyst; TT: thrombin time
| References | ▴Top |
- Cetinkaya SE, Pabuccu EG, Ozmen B, Dokmeci F. Recurrent massive hemoperitoneum due to ovulation as a clinical sign in congenital afibrinogenemia. Acta Obstet Gynecol Scand. 2011;90(2):192-194.
doi pubmed - Lee JK, Bodur S, Guido R. The management of gynecological hemoperitoneum found to be associated with a ruptured corpus luteum cyst. Gynecol Surg. 2016;13(4):305-311.
doi - Hallatt JG, Steele CH, Jr., Snyder M. Ruptured corpus luteum with hemoperitoneum: a study of 173 surgical cases. Am J Obstet Gynecol. 1984;149(1):5-9.
doi - Lucey BC, Varghese JC, Soto JA. Spontaneous hemoperitoneum: causes and significance. Curr Probl Diagn Radiol. 2005;34(5):182-195.
doi pubmed - Acharya SS, Dimichele DM. Rare inherited disorders of fibrinogen. Haemophilia. 2008;14(6):1151-1158.
doi pubmed - Schneider D, Bukovsky I, Kaufman S, Sadovsky G, Caspi E. Severe ovarian hemorrhage in congenital afibrinogenemia. Acta Obstet Gynecol Scand. 1981;60(4):431.
doi pubmed - Bottini E, Pareti FI, Mari D, Mannucci PM, Muggiasca ML, Conti M. Prevention of hemoperitoneum during ovulation by oral contraceptives in women with type III von Willebrand disease and afibrinogenemia. Case reports. Haematologica. 1991;76(5):431-433.
- Castaman G, Ruggeri M, Rodeghiero F. Congenital afibrinogenemia: successful prevention of recurrent hemoperitoneum during ovulation by oral contraceptive. Am J Hematol. 1995;49(4):363-364.
doi pubmed - Henselmans JM, Meijer K, Haaxma R, Hew J, van der Meer J. Recurrent spontaneous intracerebral hemorrhage in a congenitally afibrinogenemic patient: diagnostic pitfalls and therapeutic options. Stroke. 1999;30(11):2479-2482.
doi pubmed - Koussi A, Economou M, Athanasiou-Metaxa M. Intra-abdominal hemorrhage due to a ruptured corpus luteum cyst in a girl with congenital afibrinogenemia. Eur J Pediatr. 2001;160(3):196.
doi pubmed - Ozdemir O, Sari ME, Kurt A, Sen E, Atalay CR. Recurrent massive haemoperitoneum associated with ruptured corpus luteum in women with congenital afibrinogenemia; case report. Turk J Obstet Gynecol. 2014;11(4):242-245.
doi pubmed - Kim JH, Jeong SY, Cho DH. Massive hemoperitoneum due to a ruptured corpus luteum cyst in a patient with congenital hypofibrinogenemia. Obstet Gynecol Sci. 2015;58(5):427-430.
doi pubmed - Collen D, Tytgat GN, Claeys H, Piessens R. Metabolism and distribution of fibrinogen. I. Fibrinogen turnover in physiological conditions in humans. Br J Haematol. 1972;22(6):681-700.
doi pubmed - de Moerloose P, Neerman-Arbez M. Congenital fibrinogen disorders. Semin Thromb Hemost. 2009;35(4):356-366.
doi pubmed - Peyvandi F, Haertel S, Knaub S, Mannucci PM. Incidence of bleeding symptoms in 100 patients with inherited afibrinogenemia or hypofibrinogenemia. J Thromb Haemost. 2006;4(7):1634-1637.
doi pubmed - Lak M, Keihani M, Elahi F, Peyvandi F, Mannucci PM. Bleeding and thrombosis in 55 patients with inherited afibrinogenaemia. Br J Haematol. 1999;107(1):204-206.
doi pubmed - Ehmann WC, al-Mondhiry H. Congenital afibrinogenemia and splenic rupture. Am J Med. 1994;96(1):92-94.
doi - Girolami A, Lombardi AM, Candeo N, Scarparo P, Paternoster A. Control of ovulation-induced hemoperitoneum by oral contraceptives in a patient with congenital hypoprothrombinemia and in another with congenital factor V deficiency. Acta Haematol. 2008;119(4):236-240.
doi pubmed - Gupta N, Dadhwal V, Deka D, Jain SK, Mittal S. Corpus luteum hemorrhage: rare complication of congenital and acquired coagulation abnormalities. J Obstet Gynaecol Res. 2007;33(3):376-380.
doi pubmed - Payne JH, Maclean RM, Hampton KK, Baxter AJ, Makris M. Haemoperitoneum associated with ovulation in women with bleeding disorders: the case for conservative management and the role of the contraceptive pill. Haemophilia. 2007;13(1):93-97.
doi pubmed - Gomez A, Lucia JF, Perella M, Aguilar C. Haemoperitoneum caused by haemorrhagic corpus luteum in a patient with type 3 von Willebrand's disease. Haemophilia. 1998;4(1):60-62.
doi pubmed - Hackethal A, Ionesi-Pasacica J, Kreis D, Litzlbauer D, Tinneberg HR, Oehmke F. Feasibility of laparoscopic management of acute haemoperitoneum secondary to ruptured ovarian cysts in a haemodynamically unstable patient. Minim Invasive Ther Allied Technol. 2011;20(1):46-49.
doi pubmed - Ho WK, Wang YF, Wu HH, Tsai HD, Chen TH, Chen M. Ruptured corpus luteum with hemoperitoneum: case characteristics and demographic changes over time. Taiwan J Obstet Gynecol. 2009;48(2):108-112.
doi - Ingram GI, McBrien DJ, Spencer H. Fatal pulmonary embolus in congenital fibrinopenia. Report of two cases. Acta Haematol. 1966;35(1):56-62.
doi pubmed - Korte W, Poon MC, Iorio A, Makris M. Thrombosis in inherited fibrinogen disorders. Transfus Med Hemother. 2017;44(2):70-76.
doi pubmed - Rizk DE, Kumar RM. Congenital afibrinogenemia: treatment of excessive menstrual bleeding with continuous oral contraceptive. Am J Hematol. 1996;52(3):237-238.
doi - Kobayashi T, Asahina T, Maehara K, Itoh M, Kanayama N, Terao T. Congenital afibrinogenemia with successful delivery. Gynecol Obstet Invest. 1996;42(1):66-69.
doi pubmed - al-Mondhiry H, Ehmann WC. Congenital afibrinogenemia. Am J Hematol. 1994;46(4):343-347.
doi pubmed - Rabe FU, Solomon E. Uber Faserstoffmangel in Blut bei einem Falle von Hamophilie. Deutsch Arch F Klin Med. 1920;132:240.
- Risak E. Die fibrinogenie. Z Klin Med. 1935;128:605.
- Kim JH, Lee SM, Lee JH, Jo YR, Moon MH, Shin J, Kim BJ, et al. Successful conservative management of ruptured ovarian cysts with hemoperitoneum in healthy women. PLoS One. 2014;9(3):e91171.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
