| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 5, Number 4, December 2016, pages 117-120
Relationship of Thyroid and Sex Hormones With Osteoarthritis in Postmenopausal Indian Women
Dipanshu Sura, b, Ratnabali Chakravortya
aMAGS Medical & Research Center, West Bengal, Kolkata 700091, India
bCorresponding Author: Dipanshu Sur, MAGS Medical & Research Center, West Bengal, Kolkata 700091, India
Manuscript accepted for publication June 16, 2016
Short title: Effects of Hormones With Osteoarthritis in Postmenopausal Women
doi: https://doi.org/10.14740/jcgo410e
| Abstract | ▴Top |
Background: Osteoarthritis (OA) is the most frequent joint disease encountered in the clinical practice and is the most common cause of movement disability in the elderly. Females are found to have more severe OA, and prevalence and incidence increase after menopause. OA affects all articular tissues and finally leads to joint failure. Many experimental and clinical studies suggest that loss of hormone especially estrogen at the time of menopause increases a woman’s risk of getting OA and use of hormone replacement therapy (HRT) did seem to be associated with not only relieving of symptoms but also reduced rate of progression of OA. The aim of this case-control study was to investigate whether the thyroid and sex hormones are associated with OA in postmenopausal Indian women.
Methods: One hundred twenty-five patients suffering from OA and 82 control subjects, all aged 45 - 65 years, were included in this study. Thyroid and sex hormones were measured in the serum by ELISA and chemiluminescent immunoassay technique.
Results: Mean serum estrogen levels in the OA and control samples were 29.53 ± 3.27 and 49.21 ± 2.18 (P < 0.0001) which were significantly lower in OA patients compared with controls. Also these patients show significant change in thyroid hormones and progesterone hormone levels when compared with control subjects.
Conclusion: The findings suggest that estrogen deficiency after menopause may contribute to developing OA in postmenopausal women. Estrogen can render help in such patients but with the current level of evidence, it cannot be recommended as a first-line treatment.
Keywords: Postmenopausal women; Osteoarthritis; Hormones replacement therapy
| Introduction | ▴Top |
Osteoarthritis (OA) is a very common chronic disease that affects all joint tissues, causing progressive irreversible damage and, finally, the failure of the joint as an organ [1]. OA is the second most common rheumatological problem and is the most frequent joint disease encountered in the clinical practice [2].
Obesity is a risk factor for development of knee arthritis [3]. Age is a major risk factor for the occurrence of OA, but the mechanism by which the age is involved in the etiology of OA is largely unknown [4]. The pathological changes that occur in OA are the result of the action of biomechanical forces coupled with multiple autocrine, paracrine and endocrine cellular events that lead to a breakdown of the normal balance in tissue turnover within the joint [5].
OA strikes women more often than men and it increases in prevalence, incidence and severity after menopause [6]. Therefore, a question arises, is menopause associated with the onset and progression of OA in women and can HRT render help in such patients.
Among the multiple physiopathological mechanisms (Fig. 1) involved in OA, those related to sex hormone control have been attracting much attention, in particular those involving estrogens [7]. Estrogen lower serum calcium and phosphate inhibit bone resorption. Hurley et al [8] found low concentration of calcitonin in patients who were on estrogen replacement therapy. Normal human osteoblast cells express progesterone receptors [9]. Chaisson et al [10] have found an association between thyroid status and chondrocalcinosis or OA. Human auricular chondrocytes produce IL-6. IL-6 production by stromal osteoblastic cells is inhibited by 17B-estradiol at transcriptional levels through receptor-mediated mechanisms. Estrogen deficiency as well as its effect on IL-6 production may also make osteoclast precursors sensitive to IL-6.
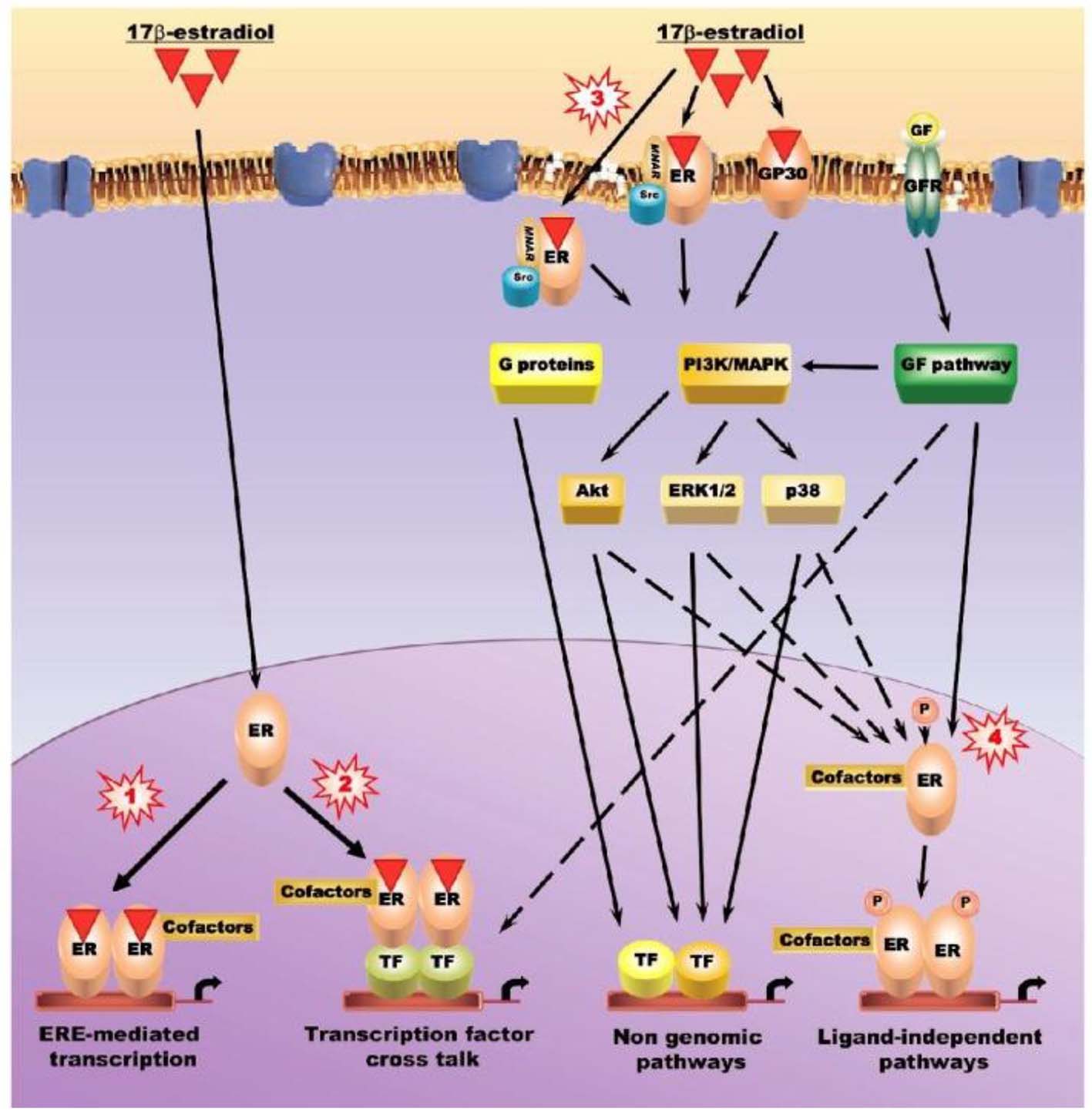 Click for large image | Figure 1. Intracellular signaling pathways used to regulate the activity of estrogens, estrogen receptors, and selective estrogen receptor modulators on articular tissues. Pathway 1: canonical estrogen signaling pathway (estrogen response element (ERE)-dependent) - ligand-activated estrogen receptors (ERs) bind specifically to EREs in the promoter of target genes. Pathway 2: non-ERE estrogen signaling pathway - ligand-bound ERs interact with other transcription factors, such as activator protein (AP)-1, NF-κB and Sp1, forming complexes that mediate the transcription of genes whose promoters do not harbor EREs. Co-regulator molecules regulate the activity of the transcriptional complexes. Pathway 3: non-genomic estrogen signaling pathways - ERs and GP30 localized at or near the cell membrane might elicit the rapid response by activating the phosphatidylinositol-3/Akt (PI3K/Akt) and/or protein kinase C/mitogen activated protein kinase (PKC/MAPK) signal transduction pathways. Pathway 4: ligand-independent pathways - ERs can be stimulated by growth factors such as insulin-like growth factor (IGF)-1, transforming growth factor-β/mothers against decapentaplegic (TGF-β/SMAD), epidermal growth factor (EGF) and the Wnt/β-catenin signaling pathway in the absence of ligands, either by direct interaction or by MAP and PI3/Akt kinase-mediated phosphorylation. Since members of these signaling pathways are transcription factors, some of them, such as SMADs 3/4, can elicit estrogen responses by interacting with ER in the non-ERE-dependent genomic pathway. ERK: extracellular signal regulated kinase; GF: growth factor; GFR: growth factor receptor; MNAR: modulator of non-genomic action of estrogen receptors; TF: transcription factor. |
The present study was aimed to reveal the fact that whether thyroid and sex hormones are associated with OA in postmenopausal Indian women.
| Materials and Methods | ▴Top |
The OA patients aged 45 - 65 years were selected from Obstetrics and Gynecology Outpatient Department of MAGS Medical & Research Center, West Bengal, Kolkata. The study was performed in accordance with ethical standards; a written informed consent was obtained from all the participants. The history was recorded by questionnaire. Patients were selected, having symptoms of OA, such as pain, stiffness, soreness, aching, discomfort, swelling and tenderness. Radiographs were taken for assessment of severity of OA. Patients taking any hormone replacement therapy (HRT), non-steroidal anti-inflammatory drugs (NSAIDs), having metabolic disease, rheumatoid arthritis (RA), joint, and systemic lupus erythromatosis (SLE), were excluded from the study. Control postmenopausal women were selected having no sign of OA clinically. Body mass index (BMI) was calculated of all patients and control subjects. Blood samples from OA female patients and control subjects were collected. About 5 mL blood was collected in centrifuge tubes. Serum was separated by centrifugation and was kept at -80 °C till the further analysis of biochemical parameters. Progesterone (P4), estradiol (E2) and calcitonin (CT) were analyzed by ELISA (IDS, Boldon, UK) and chemiluminescent immunoassay (Simens DPC Buhlmann, Salzburg, Austria) technique.
Statistical analysis
Statistical analysis was performed using the SPSS 20.0 package (SPSS, Inc., Chicago, IL). Data were expressed as the mean ± SD. The comparisons between patients and controls were performed using a t-test for unpaired data. Significance was set at 5%.
| Results | ▴Top |
A total of 125 female postmenopausal OA patients were included in the study with a mean age of 55.4 ± 4.91 years and 82 control subjects with a mean age of 52.3 ± 5.63 years (P ≤ 0.0001). Table 1 shows the age and BMI of controls and OA subjects. Significant differences were found between BMI of OA patients and control subjects (P ≤ 0.0001).
 Click to view | Table 1. Comparison of Anthropometric Characteristics Between Cases and Controls |
Table 2 shows the levels of estrogen (E2), progesterone (P4), thyroid and calcitonin, hormones in patients and control groups. E2, P4, T3, T4 and calcitonin hormones were statistically decreased (P < 0.001) in patients as compared with control subjects. There was no statistically significant values observed in triglycerides levels (P = 0.1131). 25(OH)D levels also decreased at OA patients compared to control (P ≤ 0.0001).
 Click to view | Table 2. Comparison of Biochemical Profile Between Cases and Controls |
| Discussion | ▴Top |
OA is a common age-related disorder, which is present in more than 10% of the persons older than 65 years [11]. OA may cause joint pain, bony or soft tissue swelling, tenderness, bony crepitus, peri-articular muscle atrophy, bony hypertrophy, deformity and marked loss of joint motion. It commonly affects the hands, feet, spine, and large weight-bearing joints, such as the hips and knees. It can present as localized, generalized or as erosive OA. Females are found to have more severe OA, more number of joints is involved, and have more symptoms and increased hand and knee OA. These observations and others reporting a painful form of hand OA after the menopause suggest that loss of estrogen at the time of menopause increases a woman’s risk of getting OA [12]. Polyarticulars OA also has strong female inheritance, frequent onset around menopause and an association with previous hysterectomy and gynecological surgery leading to suggestion that hormonal factors are important in this subgroup [13]. A large epidemiological study was conducted in Italy, and gave epidemiological support to the hypothesis that estrogen deficiency may increase the risk of OA [14]. In a prospective cohort study, use of estrogen replacement therapy did seem to be associated with a reduced rate of progression of knee OA [15]. Similarly long-term HRT increases bone mineral density in women who have experienced natural menopause, and protects against bone loss in surgically postmenopausal women [16]. Combined estrogen and progestin replacement therapy can relieve the knee OA symptoms of postmenopausal women. Significant differences on pain at night and tenderness around knee were seen in the treatment group compared with the control group after 1 month of treatment [17].
In the present study, there was a significant decrease in the level of estrogen and progesterone in postmenopausal women patients as compared to postmenopausal control women. Thyroid hormones are essential for the development, growth and metabolism of tissue, including bone. It is known that the action of thyroid hormones on bone tissue can be direct [18].
OA is a disease in which there is no balance between synthesis and degradation of collagen and evidences tell us that thyroid hormones fail to inhibit collagen synthesis. Present study show changes in the levels of thyroid hormone in the OA patients as compared to control subjects (Table 2). Evidences tell us that thyroid hormones fail to inhibit collagen synthesis [19].
Conclusion
In conclusion, menopause is associated with the onset and progression of OA in women. Our findings suggest that hormones, like estrogen deficiency after menopause may develop the disease of OA in postmenopausal women.
Acknowledgments
We thank the study participants. We are grateful to Amal Mallick and Joysree Mukherjee for their invaluable assistance in collecting the blood samples.
Author Contributions
DS carried out the data collection, participated in the data analysis and drafted the manuscript. RC helped in study design, diagnosis of study groups and preparing the manuscript.
Competing Interests
The authors declare that they have no competing interests.
| References | ▴Top |
- Brandt KD, Radin EL, Dieppe PA, van de Putte L. Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis. 2006;65(10):1261-1264.
doi pubmed - Chopra A, Patil J, Bilampelly V. The bhigwan (India) COPCORD: Methodology and first information report. APLAR J Rheumatol. 1997;1:145-154.
- Ding C, Cicuttini F, Scott F, Cooley H, Jones G. Knee structural alteration and BMI: a cross-sectional study. Obes Res. 2005;13(2):350-361.
doi pubmed - Papavasiliou K, Kapetanos G, Kenanidis E, Kirkos J, Potoupnis M, Sayegh F. Modifications in the serum level of intact-parathyroid hormone in a group of postmenopausal women suffering from end-stage knee osteoarthritis undergoing total knee arthroplasty compared with a control group of women undergoing elective non-orthopaedic operations. J Bone Joint Surg Br. 2010;92(B no. SUPP IV):610-611.
- Brandt KD, Dieppe P, Radin EL. Etiopathogenesis of osteoarthritis. Rheum Dis Clin North Am. 2008;34(3):531-559.
doi pubmed - Felson DT. The epidemiology of knee osteoarthritis: results from the Framingham Osteoarthritis Study. Semin Arthritis Rheum. 1990;20(3 Suppl 1):42-50.
doi - Gokhale JA, Frenkel SR, Dicesare PE. Estrogen and osteoarthritis. Am J Orthop (Belle Mead NJ). 2004;33(2):71-80.
pubmed - Hurley DL, Tiegs RD, Barta J, Laakso K, Heath H, 3rd. Effects of oral contraceptive and estrogen administration on plasma calcitonin in pre- and postmenopausal women. J Bone Miner Res. 1989;4(1):89-95.
doi pubmed - Komm BS, Terpening CM, Benz DJ, Graeme KA, Gallegos A, Korc M, Greene GL, et al. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science. 1988;241(4861):81-84.
doi pubmed - Chaisson CE, McAlindon TE, Felson DT, Naimark A, Wilson PW, Sawin CT. Lack of association between thyroid status and chondrocalcinosis or osteoarthritis: the Framingham Osteoarthritis Study. J Rheumatol. 1996;23(4):711-715.
pubmed - Ameye LG, Young MF. Animal models of osteoarthritis: lessons learned while seeking the "Holy Grail". Curr Opin Rheumatol. 2006;18(5):537-547.
doi pubmed - Felson DT, Nevitt MC. The effects of estrogen on osteoarthritis. Curr Opin Rheumatol. 1998;10(3):269-272.
doi - Doherty M, Jones A, Cawston T. Osteoarthritis. In: Isenberg DA, et al. Oxford Textbook of Rheumatology, 3rd Ed. Oxford University Press. 2004; pp. 1091-1118.
- Parazzini F. Menopausal status, hormone replacement therapy use and risk of self-reported physician-diagnosed osteoarthritis in women attending menopause clinics in Italy. Maturitas. 2003;46(3):207-212.
doi - Zhang Y, McAlindon TE, Hannan MT, Chaisson CE, Klein R, Wilson PW, Felson DT. Estrogen replacement therapy and worsening of radiographic knee osteoarthritis: the Framingham Study. Arthritis Rheum. 1998;41(10):1867-1873.
doi - Castelo-Branco C, Figueras F, Sanjuan A, Pons F, Vicente JJ, Vanrell JA. Long-term postmenopausal hormone replacement therapy effects on bone mass: differences between surgical and spontaneous patients. Eur J Obstet Gynecol Reprod Biol. 1999;83(2):207-211.
doi - Song YJ, Lin SQ, Wu ZH, Weng XS, Qiu GX, Chen FL. [Effect of combined continued hormone replacement therapy on knee osteoarthritis symptom of postmenopausal women]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004;26(5):571-575.
pubmed - Rizzoli R, Poser J, Burgi U. Nuclear thyroid hormone receptors in cultured bone cells. Metabolism. 1986;35(1):71-74.
doi - Canalis E. Effect of insulinlike growth factor I on DNA and protein synthesis in cultured rat calvaria. J Clin Invest. 1980;66(4):709-719.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
