| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 8, Number 1, March 2019, pages 21-24
Annual Cervical Cancer Screening Revealed Early Stage Clear Cell Adenocarcinoma of the Uterine Cervix: A Case Report
Mai Temukaia, b, Yoshimi Tokugawaa, Takeshi Hisamatsua, Yuri Kaminoa, Ayuko Otoshia, Chikako Tsukaharaa, Takashi Miyatakea, Hiromi Tsujic, Hironao Yasuokac, Masahiko Tsujimotoc, Tomomi Egawa-Takataa, d, Yukihiro Nishioa
aDepartment of Obstetrics and Gynecology, Osaka Police Hospital, Osaka, Japan
bDepartment of Obstetrics and Gynecology, Aizenbashi Hospital, Osaka, Japan
cDepartment of Diagnostic Pathology, Osaka Police Hospital, Osaka, Japan
dCorresponding Author: Tomomi Egawa-Takata, Department of Obstetrics and Gynecology, Osaka Police Hospital, 10-31 Kitayama-cho, Tennoji-ku, Osaka 543-0035, Japan
Manuscript submitted January 8, 2019, accepted February 20, 2019
Short title: Clear Cell Carcinoma of the Uterine Cervix
doi: https://doi.org/10.14740/jcgo530
| Abstract | ▴Top |
Clear cell adenocarcinoma of the uterine cervix (CCAC) is rare. We describe a case of CCAC, which was treated with complete resection without adjuvant therapy. The patient was a 42-year-old woman, who had no chief complaint. She had undergone annual cervical cancer screening, and no abnormalities had been detected in previous examinations. However, in a subsequent screening examination, cervical cytology produced negative results, but cervicovaginal erosion was observed at the uterine cervix. A detailed examination led to adenocarcinoma being suspected, and so conization was performed, resulting in a diagnosis of CCAC. A microscopic examination revealed atypical cells with clear-slightly basophilic cytoplasm, and immunohistochemical studies showed that they were positive for napsin A, p16, and AE1/3. Radical hysterectomy, pelvic lymph node dissection, and bilateral salpingo-oophorectomy were performed. Complete resection was achieved, and the postoperative diagnosis was CCAC, pT1b1N0M0, ly(-), v(-). The patient did not undergo adjuvant treatment. She is still alive and recurrence-free.
Keywords: Clear cell carcinoma; Cervical cancer; Screening
| Introduction | ▴Top |
Clear cell adenocarcinoma of the cervix (CCAC) is a rare type of gynecological cancer, which accounts for about 0.48% of cervical carcinomas and 4-9% of cervical adenocarcinomas [1, 2]. Exposure to diethylstilbestrol (DES) in utero due to maternal use of the drug is a risk factor for CCAC among young females [3]. CCAC often produces negative findings during routine vaginal cytology, so its early detection can be difficult. It has been reported that the prognosis of adenocarcinoma of the uterine cervix is worse than that of squamous cell carcinoma (SCC) of the uterine cervix, but there have not been any prospective studies about the postoperative adjuvant treatment of CCAC.
We experienced a case of CCAC, which was diagnosed after several years of normal cytological findings and was successfully treated via complete resection without adjuvant therapy. We report this case together with a brief review of the related literature.
| Case Report | ▴Top |
The patient was a 42-year-old woman (gravida 0) without any coexisting disease. She had experienced sexual intercourse. She had no complaints related to gynecological disease. She had undergone cervical cancer screening every year at her own expense as part of health checkups conducted at a clinic, but no abnormalities had been found during previous examinations. However, during a subsequent cervical cancer screening examination, cervical cytology produced negative results for intraepithelial lesions or malignancy, but cervicovaginal erosion was observed at the uterine cervix. Therefore, she was referred to our hospital for consultation.
A vaginal examination showed cervicovaginal erosion, but no abnormalities were seen on a transvaginal ultrasonogram. However, atypical glandular cells were detected during cervical cytology, and so colposcopy was conducted, which revealed atypical blood vessels (Fig. 1a). A cervical biopsy of the region containing the atypical blood vessels resulted in a diagnosis of suspected adenocarcinoma. Endometrial cytology was negative for malignancy. The patient’s serum levels of tumor markers were all within the normal range (carcinoembryonic antigen: 1.0 ng/mL, SCC antigen: 0.5 ng/mL, cancer antigen 125 (CA125): 18 U/mL, CA19-9: 9 U/mL). Pelvic magnetic resonance imaging (MRI) did not reveal a tumor (Fig. 1b), and no metastatic lesions were detected on thoracic or abdominal computed tomography. We performed conization for diagnostic purposes. A microscopic examination of the surgical specimen revealed atypical multiplying and infiltrating flattened and cuboidal cells with clear-slightly basophilic cytoplasm, arranged in irregular tubular structures (Fig. 2a-c). Microscopically, the tumor cells had invaded the cervix to a depth of 9 mm. The cytoplasm of the tumor cells was positively stained with periodic acid-Schiff (PAS) stain, whereas it was not stained with diastase-digested-PAS (di-PAS) (Fig. 3a, b). Thus, the cytoplasm was shown to contain glycogen. Immunohistochemical studies demonstrated that the tumor cells were positive for napsin A, p16, and AE1/3 and negative for CD10, calretinin, the estrogen receptor, the progesterone receptor, and D2-40. The Ki-67 index of the tumor cells was almost 5% (Fig. 3c). Thus, the conization resulted in a pathological diagnosis of clear cell adenocarcinoma. The cut end of the pathological sample was positive. A histological examination of a tissue sample obtained via endometrial curettage did not show any evidence of malignancy.
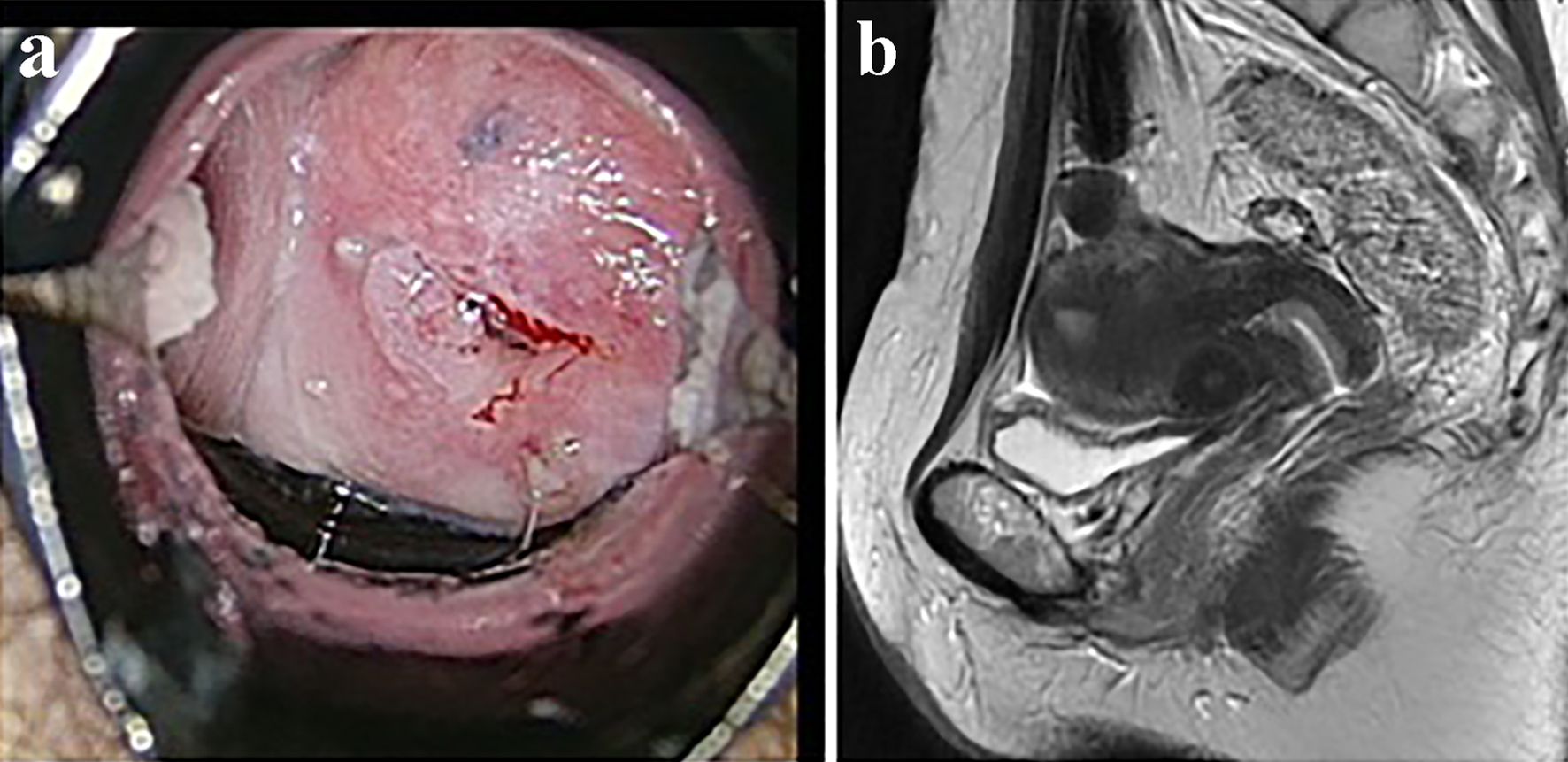 Click for large image | Figure 1. The results of imaging examinations. (a) Colposcopic findings. Atypical vessels were detected on colposcopy. (b) T2-weighted pelvic MRI images. No tumor was detected. |
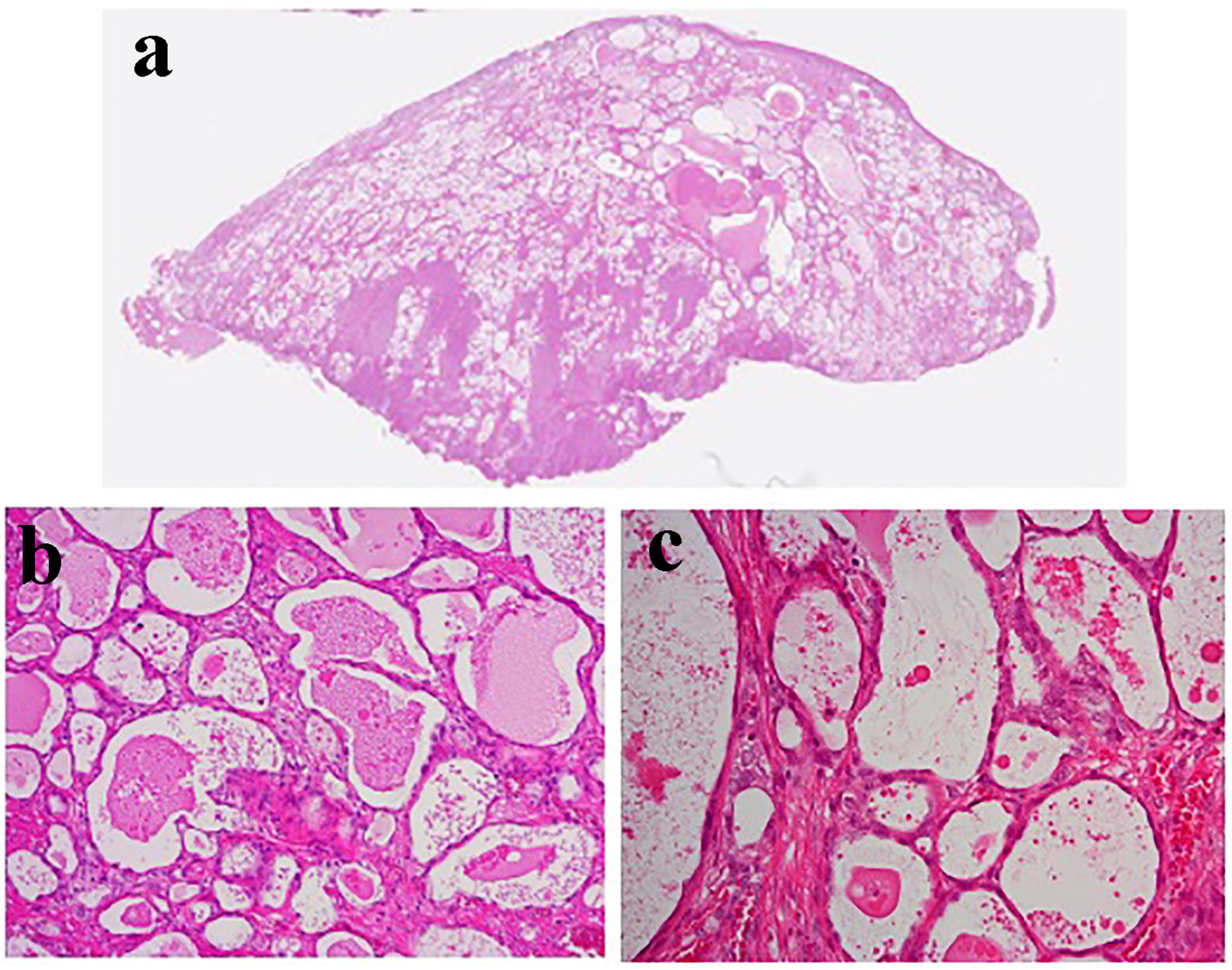 Click for large image | Figure 2. The histological findings of the conization specimen. Atypical flattened and cuboidal cells filled with eosinophilic secretions had formed dilated and irregular lumens. The tumor cells had invaded the cervix to a depth of 9 mm. (a) Magnification: × 1. (b) Magnification: × 4. (c) Magnification: × 20. |
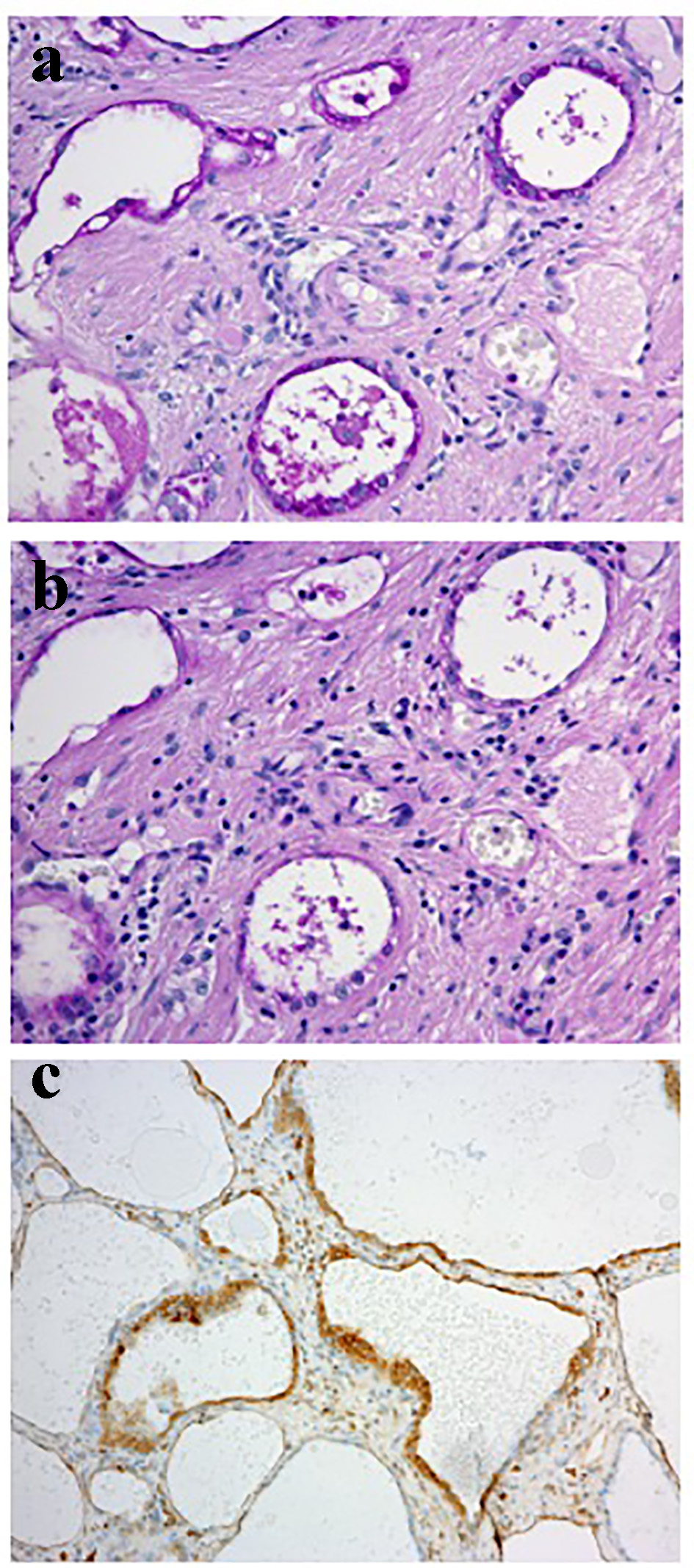 Click for large image | Figure 3. The results of PAS staining and the immunohistochemical staining of napsin A. (a) PAS staining. The tumor cells were positively stained with PAS (magnification: × 20). (b) The di-PAS staining (magnification: × 20). The tumor cells produced negative results during di-PAS staining, which indicated that they contained glycogen. (c) Immunohistochemical staining of napsin A (magnification: × 10). The tumor cells were positive for napsin A. |
The patient was preoperatively diagnosed with CCAC (International Federation of Gynecology and Obstetrics (FIGO) stage IB1 cervical cancer). Radical abdominal hysterectomy combined with bilateral salpingo-oophorectomy and systemic pelvic lymphadenectomy were performed as primary treatments for the cervical cancer. The microscopic findings of the surgical specimen included invasion into the muscular layer, while atypical cuboidal cells with oval swollen nuclei had formed irregular tubular structures. The surgical stump was negative, and there was no metastasis in the ovaries or lymph nodes. There was a small amount of aberrant endometrial tissue in the connective tissue of the cervix.
The postoperative diagnosis was clear cell adenocarcinoma of the uterine cervix, pT1b1N0M0, ly(-), v(-). As we achieved a complete surgical resection, and there was no residual disease, the patient did not receive any other adjuvant therapy, chemotherapy, or radiotherapy after the operation. Currently, two years have passed without recurrence.
| Discussion | ▴Top |
CCAC of the cervix is a rare type of tumor, accounting for about 0.48% of cervical carcinomas and 4-9% of cervical adenocarcinomas [1, 2]. The risk factors for and pathogenesis of CCAC remain unclear. However, it is apparent that exposure to DES in utero due to maternal use of the drug is a risk factor for CCAC. DES was used to prevent miscarriages and other complications of pregnancy in the United States between 1938 and 1971. In 1971, the use of DES in pregnant women was prohibited because it was found to carry an increased risk of clear cell adenocarcinoma in female offspring [3]. During the period when DES was used in pregnant women, cases of CCAC involving younger ages at onset were seen, but in the post-DES era, DES is not used in pregnant women, and the frequency of CCAC is higher in the postmenopausal period [4]. There have not been any DES-related cases of CCAC in Japan. Instead, in Japan CCAC normally arises in middle-aged and elderly women.
The typical symptoms of CCAC include prolonged genital bleeding, as is the case with other histological types of cervical cancer [2, 5]. However, in the early stages of CCAC, there may be no symptoms. Also, CCAC frequently produces negative results during cervical cytology, and CCAC often cannot be palpated [6, 7]. Cytological examinations have been found to be less useful for diagnosing cervical adenocarcinoma than SCC [5, 8]. In our case, although the first cytological examination produced a negative result, the detection of cervicovaginal erosion resulted in a diagnosis of CCAC.
The pathological features of CCAC include the proliferation of cells with clear cytoplasm arranged in papillate, tubular, and/or solid growth patterns, and the appearance of hobnail cells, whose nuclei project into the lumen. These features are similar to those of ovarian clear cell carcinoma. As is the case in ovarian clear cell carcinoma, napsin A has been found to be a marker of CCAC [9]. In our case, the tumor was positive for napsin A.
The pathogenesis of CCAC is unclear, but a previous case report suggested that, as is found in ovarian clear cell carcinoma, endometriosis might be involved in the pathogenesis of CCAC [10]. In the current case, aberrant endometrial tissue was observed in the uterine cervix.
The prognostic factors for CCAC include a tumor size of > 4 cm, the presence of lymph node metastasis, and advanced stage carcinoma [2, 5, 8]. In a study by Thomas et al involving 34 facilities [5], although it was suggested that early stage CCAC patients might be at slightly higher risk of nodal spread, clear cell histology by itself did not appear to denote a worse prognosis than SCC of the cervix in the absence of traditional risk factors. In addition, the stage I or IIA patients (n = 26) demonstrated superior 3-year overall survival (OS) compared with the advanced stage (n = 8) patients (91% vs. 22%, P < 0.001). Furthermore, the presence of positive lymph nodes had adverse effects on 5-year progression-free survival and 5-year OS among stage I and IIA CCAC patients.
The established treatment for early stage CCAC is surgery [6, 11]. As for postoperative adjuvant treatments, chemotherapy or concurrent chemoradiotherapy are used at some facilities [12, 13], but there have not been any prospective studies about the use of such adjuvant treatments for CCAC. Thomas et al suggested that patients with low-risk early stage CCAC that are not treated with adjuvant therapy exhibit good prognoses, so they can be managed with radical surgery alone [5, 13, 14].
In the current case, the disease was diagnosed at an early stage (after the gynecologist that conducted her cervical cancer screening noticed slight erosion) because the patient underwent annual cervical cancer screening examinations at her own expense (although there is governmental support for biennial cervical cancer screening in Japan).
Conclusion
We experienced a rare case of CCAC. The patient had exhibited normal cytological findings for several years, but slight erosion of the cervix was subsequently observed, which resulted in a diagnosis of CCAC.
Conflict of Interest
The authors declare no conflict of interest.
Abbreviations
CA: cancer antigen; CCAC: clear cell adenocarcinoma of the uterine cervix; DES: diethylstilbestrol; di-PAS: diastase-digested PAS; FIGO: International Federation of Gynecology and Obstetrics; MRI: magnetic resonance imaging; PAS: periodic acid-Schiff; SCC: squamous cell carcinoma
| References | ▴Top |
- Noller KL, Decker DG, Dockerty MB, Lanier AP, Smith RA, Symmonds RE. Mesonephric (clear cell) carcinoma of the vagina and cervix. A retrospective analysis. Obstet Gynecol. 1974;43(5):640-644.
pubmed - Reich O, Tamussino K, Lahousen M, Pickel H, Haas J, Winter R. Clear cell carcinoma of the uterine cervix: pathology and prognosis in surgically treated stage IB-IIB disease in women not exposed in utero to diethylstilbestrol. Gynecol Oncol. 2000;76(3):331-335.
doi pubmed - Schrager S, Potter BE. Diethylstilbestrol exposure. Am Fam Physician. 2004;69(10):2395-2400.
pubmed - Kaminski PF, Maier RC. Clear cell adenocarcinoma of the cervix unrelated to diethylstilbestrol exposure. Obstet Gynecol. 1983;62(6):720-727.
pubmed - Thomas MB, Wright JD, Leiser AL, Chi DS, Mutch DG, Podratz KC, Dowdy SC. Clear cell carcinoma of the cervix: a multi-institutional review in the post-DES era. Gynecol Oncol. 2008;109(3):335-339.
doi pubmed - Herbst AL, Scully RE. Adenocarcinoma of the vagina in adolescence. A report of 7 cases including 6 clear-cell carcinomas (so-called mesonephromas). Cancer. 1970;25(4):745-757.
doi - Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284(15):878-881.
doi pubmed - Jiang X, Jin Y, Li Y, Huang HF, Wu M, Shen K, Pan LY. Clear cell carcinoma of the uterine cervix: clinical characteristics and feasibility of fertility-preserving treatment. Onco Targets Ther. 2014;7:111-116.
doi pubmed - Talia KL, Wong RW, McCluggage WG. Expression of markers of Mullerian clear cell carcinoma in primary cervical and vaginal gastric-type adenocarcinomas. Int J Gynecol Pathol. 2018.
doi pubmed - Hiromura T, Tanaka YO, Nishioka T, Satoh M, Tomita K. Clear cell adenocarcinoma of the uterine cervix arising from a background of cervical endometriosis. Br J Radiol. 2009;82(973):e20-22.
doi pubmed - Chan KK, Ip P, Kwong P, Tam KF, Ngan HY. A combination of chemoirradiation and chemotherapy for treatment of advanced clear cell adenocarcinoma of the cervix. Int J Gynecol Cancer. 2008;18(3):559-563.
doi pubmed - Seki H, Takada T, Sodemoto T, Hoshino H, Saitoh K, Uekusa T. A young woman with clear cell adenocarcinoma of the uterine cervix. Int J Clin Oncol. 2003;8(6):399-404.
doi pubmed - Tantitamit T, Hamontri S, Rangsiratanakul L. Clear cell adenocarcinoma of the cervix in second generation young women who are without maternal exposure to diethylstilbestrol: A case report. Gynecol Oncol Rep. 2017;20:34-36.
doi pubmed - Lee YL, Chang KH, Kyung MS, Kim HB, Park SH. A case of clear cell adenocarcinoma in the uterine cervix of 52-year-old virgin. Korean J Obstet Gynecol. 2012;55(3):192-196.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
