| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 9, Number 3, September 2020, pages 60-69
Bilateral Tubal Pregnancy Following Spontaneous Conception: A Case Report and Literature Review
Mohamad K. Ramadana, b, Mariam Kharroubia, b, Rawia Bou-Ghanema, c, Dominique A. Badrb, d, e
aDepartment of Obstetrics and Gynecology, Rafic Hariri University Hospital, Beirut, Lebanon
bDepartment of Obstetrics and Gynecology, Lebanese University, Beirut, Lebanon
cDepartment of Obstetrics and Gynecology, Beirut Arab University, Beirut, Lebanon
dDepartment of Obstetrics and Gynecology, Brugmann University Hospital, Universite Libre de Bruxelles, Brussels, Belgium
eCorresponding Author: Dominique A. Badr, Department of Obstetrics and Gynecology, Brugmann University Hospital, Universite Libre de Bruxelles, Brussels, Belgium
Manuscript submitted April 22, 2020, accepted June 26, 2020, published online September 9, 2020
Short title: Spontaneous Bilateral Tubal Pregnancy
doi: https://doi.org/10.14740/jcgo642
| Abstract | ▴Top |
Ectopic tubal pregnancy (ETP) continues to be a serious health condition and the leading cause of maternal morbidity and mortality in early pregnancy. Its incidence has increased lately to reach 1.5-2% of all early pregnancies. Bilateral tubal pregnancy (BTP) is the rarest form of extra-uterine pregnancy. It is usually associated with infertility treatment, while spontaneous occurrence is exceptionally rare. The clinical presentation of BTP is unpredictable and the performance of currently available imaging modalities is unsatisfactory, rendering the diagnosis extremely difficult, mostly made during surgery. Herein, we present a case of a 39-year-old patient who presented with a disturbed ectopic pregnancy. Intra-operatively, a ruptured tubal pregnancy, together with another intact ETP in the contralateral tube, was encountered. Salpingectomy and salpingostomy were done respectively preserving the intact tube with uneventful recovery. This was followed by a review of recent literature on BTP. Definitive diagnosis of BTP continues to be made at surgery in spite of some improvement in preoperative detection. Meticulous examination by sonographers of the whole pelvis in early pregnancy should be the routine even in extra-uterine pregnancies. Gynecologists should explore contralateral tube while performing surgery on ETP. Furthermore, close surveillance with clinical, sonographic and serial serum beta-chain human chorionic gonadotropin, should be implemented for all ETP even following salpingectomy.
Keywords: Bilateral ectopic pregnancy; Non-simultaneous ectopic pregnancy; Simultaneous ectopic pregnancy; Salpingectomy; Salpingostomy
| Introduction | ▴Top |
Ectopic pregnancy (EP) is defined as the implantation and development of the blastocyst anywhere outside the endometrial cavity. The incidence of EP in developed countries has increased in the past 30 years to reach approximately 2% of all pregnancies in 1992 [1]. This was attributed to the increase in sexual transmitted infections (STIs), intrauterine device (IUD) use, surge in tubal surgery and the wide-spread use of assisted reproductive techniques (ARTs). In spite of the immense improvement in the detection of EP with the introduction of transvaginal ultrasound (TVUS) and beta-chain human chorionic gonadotropin (beta-hCG) and the decrease of almost 90% in related mortality rates, EP is still responsible for about 9% of deaths that follow all early pregnancies in USA [1]. While unilateral tubal pregnancy constitutes 90% of all EPs, bilateral tubal pregnancy (BTP) is considered the rarest form [2]. Its exact frequency is difficult to estimate with accuracy depending on case reports, yet, the most reported incidence was 1 in 200,000 pregnancies [3]. This represents 1/750 - 1,500 of all EPs [4]. Commonly, there is a history of ART preceding the development of BTP, whereas spontaneous occurrence of this condition, without ovulation induction, is extremely rare. Hereby, we describe the clinical findings of a case with spontaneous BTP. Then, we present a brief review of recent literature (January 2007 - March 2019) for reports on spontaneous BTP published in English language.
| Case Report | ▴Top |
This was a 39-year-old woman, G4P1A2 with previous one normal spontaneous vaginal delivery and two evacuation and curettages done for first trimester incomplete abortions. The index pregnancy was spontaneous and the patient claimed to be at 10 weeks’ gestation. She has had non-significant past medical and surgical history and was an occasional smoker but denied any history or treatment for STI, use of IUD, chronic pelvic pain or abnormal vaginal secretions.
She presented to the emergency department (ED) complaining of diffuse colicky abdominal pain that became constant with increasing intensity. It started several hours prior to presentation and was associated with multiple episodes of vomiting and abdominal distension with obstipation. She had stable vital signs with blood pressure of 110/70 mm Hg and pulse of 90 beats per minute. Beta-hCG was 17,229 IU/L. An abdominal computed tomography (CT) scan was ordered by the ED team and showed an empty uterus with thin endometrium with hemoperitoneum and a left-sided complex adnexal mass suggestive of disturbed left ectopic tubal pregnancy (ETP). These findings were confirmed by TVUS done by the gynecology team. At laparotomy, the patient was found to have a massive hemoperitoneum of about 1 L and a ruptured extensively damaged left ETP for which salpingectomy was performed. Exploration of the contralateral side revealed a blueish vascularized ampullary mass distending the right tube and consistent with another EP (Fig. 1). Linear salpingostomy by cold knife was done and tissues were sent in two separate containers for examination. Hemostasis of the tube was done as appropriate.
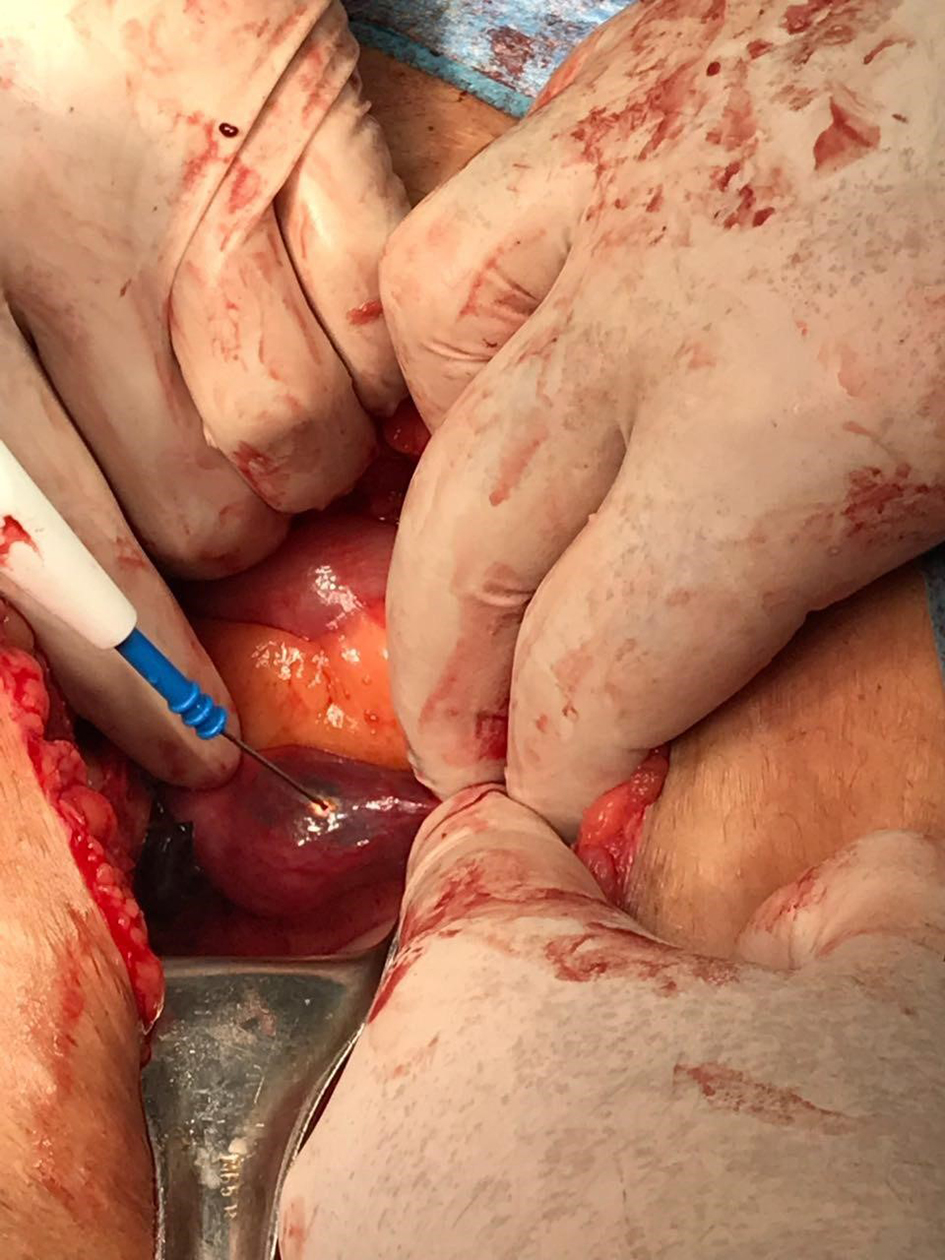 Click for large image | Figure 1. Image showing incidental intra-operative findings of right-sided dilated fallopian tube containing trophoblastic tissues. |
Serum beta-hCG level on third postoperative day was 2,546 IU/L. The patient was instructed to repeat beta-hCG weekly till undetectable value is attained. Histo-pathology report confirmed the presence of BTP. Approval of the hospital IRB to acquire relevant data on imaging documents and clinical findings from the hospital database was obtained and the patient consented to the use of pertinent information for scientific purposes provided personal identity is kept anonymous.
| Case Discussion | ▴Top |
This case exemplifies the typical clinical presentation of the majority of cases with BTP. The patient presented complaining of acute pelvic and abdominal pain before collapse. CT scan is considered the primary modality of imaging for patients presenting with acute abdomen in all EDs [5]. It accurately predicted the presence of massive hemoperitoneum and the presence of left adnexal mass (magma), a picture consistent with disturbed ETP. TVUS confirmed these findings; however, neither CT scan nor TVUS was capable of making the diagnosis of BTP. Definitive diagnosis of BTP was only attained by direct visualization of both ectopic sites at laparotomy. Optimal management of EP entails preventing maternal hemorrhagic complications while preserving the fertility potential of the patient. This can only be achieved with early diagnosis before tubal rupture. Although early diagnosis of EP or BTP can prevent hemorrhagic catastrophes, yet, is not an assurance that salpingectomy can be avoided, as the choice of performing this surgery depends, on the size, location of EP, condition of the fallopian tube and the desire to preserve fertility. In this case, salpingostomy was performed on the contralateral tube when its condition was considered salvageable. Exploration of the contralateral tube enabled us to discover the second EP.
| Literature Review | ▴Top |
We performed a search for the terms “spontaneous” and “bilateral” and “tubal ectopic pregnancy” in PubMed and Google Scholar database, published during the period between January 1, 2007 and March 31, 2019 in English language. The search retrieved 66 articles. After screening for relevance, only 59 case reports were deemed appropriate for analysis (including our own case). “Spontaneous” describes women with natural cycles, not receiving any form of hormonal manipulation for ovulation induction. We emphasized on analyzing clinical presentation and diagnostic findings of 59 cases (Supplementary Material 1, www.jcgo.org). Finally we proposed few ideas to improve the management and to ameliorate related complications. Two comprehensive reviews, 10 years apart [6, 7], separately identified two major groups of BTP. The first group (primary BTP) included patients who conceived spontaneously and naturally, while the second group (secondary BTP) comprised patients who developed this complication following the use of ARTs which incorporate induction of poly-ovulation. De-Los-Rios et al concluded that the underlying pathophysiology might differ between these two groups and recommended studying each group separately [7]. We limited our review to cases who conceived naturally (spontaneous BTP).
Spontaneous (non-simultaneous/asynchronous BTP)
In addition to the two major groups identified previously by Bustos and De-Los-Rios [6, 7], we could recognize another new subset of patients with non-simultaneous (asynchronous) occurrence of BTP. Among the 59 patients of this series, eight women had unilateral EP at initial presentation and this was documented by direct visualization at laparoscopy/laparotomy [8-15]. These patients later developed a second EP in the contralateral tube. In four cases, laparoscopy was used for the surgical treatment of the first EP and the contralateral tube was considered normal and healthy-looking as documented on videos [10-13]. These four patients were admitted later with acute abdomen and underwent second surgery. The other four patients were started initially on methotrexate for assumed unilateral undisturbed EP [8, 9, 14, 15]. Upon standard follow-up, new EP was then seen with TVUS in the contralateral tube. Methotrexate treatment was extended and was successful in two cases [9, 14], whereas in the remaining two, pain did not subside and the level of beta-hCG did not show any decrease, so laparoscopic surgery was performed [8, 15]. All patients denied any intercourse in the interval between the two admissions. Asynchronous occurrence of BTP is not limited to spontaneous BTP as several cases were also reported following ART [16, 17]. The diagnosis of BTP was delayed on an average of 14.4 days, and a range of 4 - 32 days. Remarkably, this delay was not accompanied with increased damage of the second tube as only one case underwent bilateral salpingectomy due to bilateral ruptured tubes.
Incidence
BTP is estimated to occur at a rate of 1:200,000 clinical pregnancies, which roughly corresponds to a rate of 1:725 - 1,580 EPs [3, 18]. The true incidence of BTP is difficult to estimate being an exceptionally rare event and the existing information is collected mostly from old case reports or series [7]. Furthermore, there could be also a tendency towards underestimation if we consider that many EPs go unnoticed, undergo tubal abortion or transform into chronic ectopics. The most recent review by De-Los-Rios et al estimated BTP at an incidence of 1:914 EPs [7].
Around 200 - 250 cases of BTP have been reported in the literature [19]. Probably, the first comprehensive review that included 76 cases was published by Fishback in 1939. He cited articles published since 1904 [18]. Albeit the increased reporting of BTP that took place over the past two decades [20-22], it is not clear whether this reflects a mere reporting bias, a real increase in the incidence or a genuine advancement in the detection of this rare condition. This involved the two forms of BTP especially that associated with the use of ART [23]. Reviews prior to 1980s indicated that spontaneous BTP constituted all cases, but later with the wide-spread use of ART; about 50% of cases with BTP were attributed to spontaneous occurrence [6, 7]. Issat et al in 2009 collected 50 cases since 1997 where 50% were spontaneous BTP [24]. Similarly, Zhu et al found that 44% of 30 BTP cases reported between 1980 and 2016 were spontaneous, and currently fewer cases are expected to be ascribed to spontaneous conception [16].
Epidemiology
ETP has well-defined risk factors that are known to inflict damage to the fallopian tube integrity. The most common are: previous EP, STI, pelvic/tubal surgery including bilateral tubal ligation (BTL), infertility and IUD users in addition to many other less significant factors. Nevertheless, in about 50% of cases, no risk factor could be identified [25]. The introduction of assisted reproduction treatment was found to be the most important risk in BTP [26]. In fact, ART introduction had deleterious effect on the incidence of ETP. While the overall incidence of ETP was 0.5-2%, with ART this became 4.5% [27]. For BTP to occur, in addition to the aforementioned factors, more specific factors are implicated in its pathogenesis, especially the use of medications intended to induce poly-ovulation in addition to the technique and number embryo transferred. Poly-ovulation whether induced by hormonal manipulation or occurring spontaneously is conditional for the occurrence of BTP. For spontaneous BTP, twinning proneness might be considered as the most important risk factor for this group. In fact, the risk for BTP among native Africans was found to be 1:50 ectopics [28].
The quality of reporting and documentation of many cases in this review was poor or incomplete. For example, STI could have been present in some patients but no chlamydia antibody titers were tested. Similarly, there was no reference to smoking in most cases, though it has been found to be an important risk factor [29]. Nevertheless, having current or a history of one or more of the following was considered by us to be a pertinent risk factor: tubal/pelvic surgery, STI, IUD, EP, infertility, cesarean delivery and abortions demanding dilation and curettage. Of the 59 cases in this review, epidemiologic data were available in 57/59. No identifiable risk factor was seen in 54.6% while 30.3% were nulliparas. The most frequent risk factors were: infertility in 10.0%, previous EP in 10.0% and STI in 8.7%. More than one risk factor was found in 15.7%.
Clinical presentation
Clinical presentation of BTP is not different from unilateral EP. This ranges from incidental diagnosis in totally asymptomatic cases presenting to the clinic with amenorrhea, positive pregnancy test and minimal discomfort or vaginal spotting to a full-blown picture of acute abdomen and hemorrhagic shock depending on the extent of tubal damage. The extent of pain or hemodynamic instability, unexpectedly, was not doubled in spite of the presence of two EPs. The median maternal age was 30 years (range 14 - 40), mean parity was 1.5 and mean gestational age at diagnosis was 7.5 weeks (range 4 - 14). Upon revision of the preoperative condition of cases in this group, disturbed ectopic with the presence of some blood in the pouch of Douglas (POD) was reported in 39/59 (66.1%). This is not to imply that all of them were in shock and critical condition. Actually only 20/59 (33.9%) cases were in hemodynamically unstable condition requiring emergent surgery. Nevertheless, this hemoperitoneum could have added to the difficulty in making the sonographic diagnosis of BTP but not that of unilateral EP.
Implication
EP is the leading cause of maternal death in the first trimester accounting for 10% of early pregnancy-related maternal mortality [1]. There are no published reports on maternal mortality secondary to BTP; yet, similar to unilateral EP, it has the capability to inflict such a complication [30]. Nonetheless, it definitely has greater potential to cause major impairment of the fertility by damaging both tubes thus rendering the patient incapable of natural conception and completely dependent on ART. Actually, 24 of the 59 patients in this series (40.6%) were treated with bilateral salpingectomy, in spite the fact that bilateral rupture was encountered only in three cases. In the majority of cases, this surgical act was performed due to the pathologic condition of the tubes while in two it was done electively (BTL) upon the request of the patients as they have completed their family [21, 31]. This 40.6% rate of bilateral salpingectomy is not far from the rate reported by Bustos-Lopez in 1998. In his review, complete surgical information was available on 30/38 cases. Bilateral salpingectomy was done in 15 cases at a rate of 50% [6].
In a couple of cases, the presence of tubal pathology in the contralateral tube was deceiving for some gynecologists who had ignored the possibility of a second EP. Actually, a second EP was discovered in four cases that had their contralateral tubes removed for edematous, distorted scarred, pyo/hematosalpinx without apparent evidence of an EP [8, 13, 32, 33]. El-Hakim et al reported a case of EP in one tube that was removed and a hydrosalpinx in the contralateral tube for which nothing was done. One week later the patient was admitted again with persistent abdominal pain and increasing beta-hCG level to discover a new EP in the pathologic tube with hydrosalpinx [8]. Similar cases of EP (not in this group) were reported to occur in preexisting hematosalpinx [34], edematous or scarred tubes explained as residual pathology from previous EP [35]. It is advisable to follow these patients closely for the high likelihood of developing another EP soon or later in subsequent pregnancies. According to El-Hakim et al, clinicians should suspect EP in patients with evidence of severe tubal disease [8]. Whether to remove contralateral pathologic tubes at the time of surgery or to be satisfied with meticulous examination of these tubes is not clear. It might be wise, though, to remove any abnormally-looking tube, as these expose the patient to future EP with all related complications, in addition to a possible risk, albeit rare, of harboring a second EP. We totally concur with Vyas et al who concluded that having an EP places the patient at increased risk of another EP not only in the future but also at the present time [36]. This implies that even when an EP was identified in a patient presenting with acute abdominal pain, a careful search for a second EP in the contralateral tube is reasonable. Moreover, even the presence of an intrauterine early pregnancy should not be a deterrent for a thorough examination of the rest of pelvis and adnexae [37]. In fact, one patient in this series had heterotopic pregnancy and the possibility of BTP was never entertained [38]. Furthermore, one case developed BTP after previous partial or segmental salpingectomy performed for BTL [39]. This was also reported in three cases in the review by Bustos-Lopez et al [6].
Diagnosis
Beta-hCG values with the corresponding gestational age were available in 26/59 cases with values ranging between 152 and 25,000 mIU/mL. These values when analyzed showed poor correlation of r = 0.132 (Fig. 2). Similar observation was made by De-Los-Rios et al where no correlation could be found between gestational age and beta-hCG levels [7]. Logically, values of beta-hCG are expected to be higher in the presence of two EPs, this however, was not consistent among all cases and was not helpful in identifying BTP. Furthermore, it could even be misleading. Observations obtained from unilateral EP cannot be extrapolated to BTP for two simple reasons. There are no beta-hCG levels that correspond to normal or abnormal pregnancies in twin gestation. Furthermore, no discriminatory levels can segregate between what is a normally ongoing and an abnormal twin gestation or BTP. This, however, does not imply to totally overlook beta-hCG levels. Actually, in a couple of cases, the high-level motivated gynecologists to scrutinize the TVUS features, searching for any abnormality in the whole pelvis even when an EP was found in one of the adnexa. On the other hand, in several cases beta-hCG levels were lower even for a single EP. Hence no reliance should be made solely on beta-hCG level to reach the diagnosis of BTP.
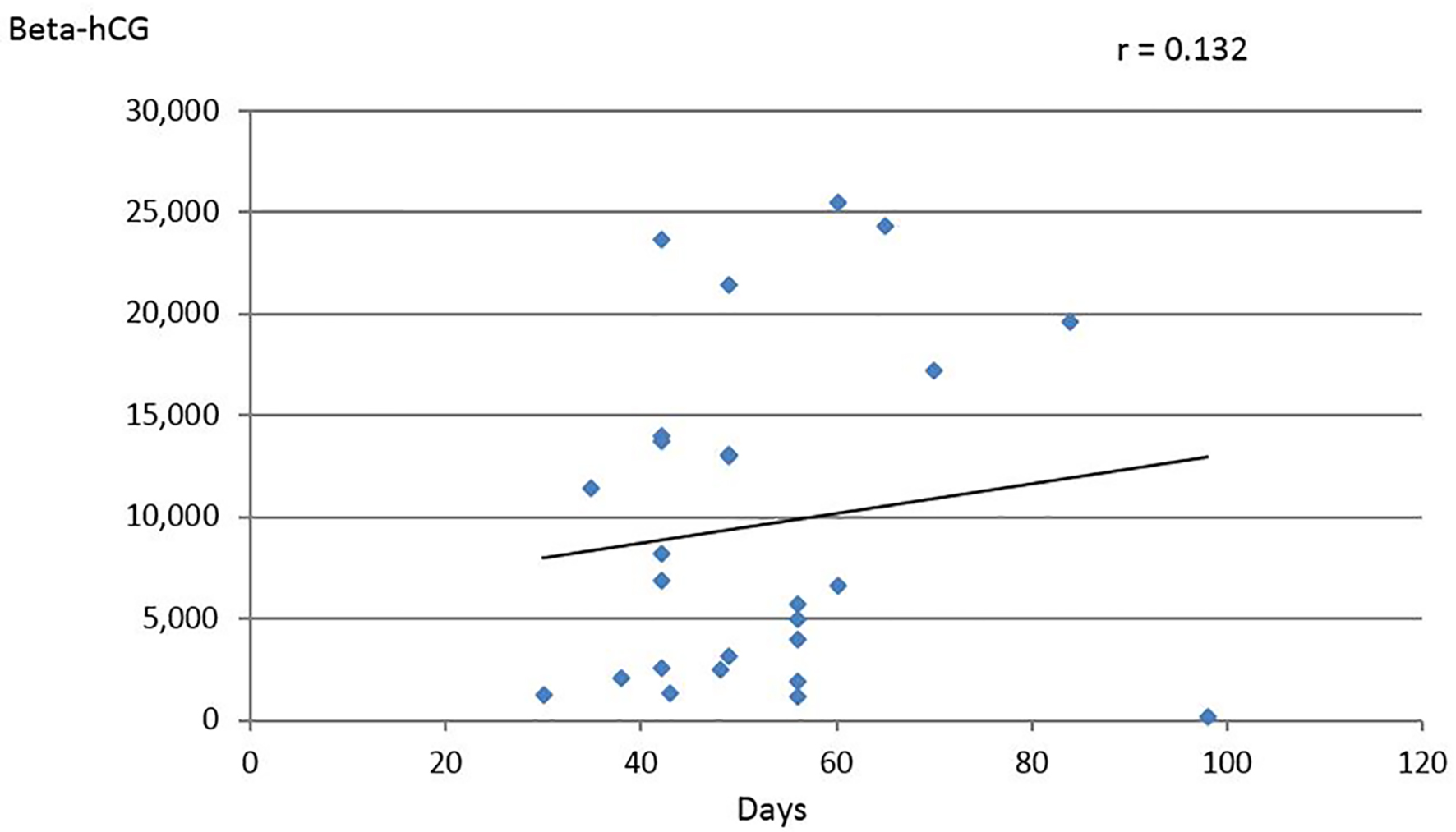 Click for large image | Figure 2. Relation of beta-hCG values (IU/L) to gestational age in days (data derived from 26 cases). |
Before the advent of ultrasonography, diagnosis relied on histopathologic identification of fetal parts and placental material in both tubes [18]. This was modified later by Norris et al who stated that it is sufficient to identify chorionic villi in each tube for the diagnosis to be made [40]. Currently, TVUS is considered the modality of choice for the diagnosis of EP. Prediction of BTP with TVUS, though, is not as clear. This can be due to the limited awareness of this condition or obscured visibility in the presence of blood clots in the pelvis.
Of the 59 cases, eight developed the asynchronous form of the disease, where, at the initial presentation to the hospital, only unilateral EP was present. Of the remaining 51 cases, a preoperative diagnosis of BTP was achieved in seven cases (13.7%) [8, 14, 16, 24, 41-43]. Three of them had evident bilateral ectopic gestational sacs, other three had adnexal masses visualized over each side and the last one had gestational sac on one side and another suspected ectopic sac inside a hematosalpinx on the opposite side. Of these seven cases, four had identifiable fetal cardiac activity in one or both tubes. In three cases, there was blood in the POD; yet, this did not hinder preoperative diagnosis of BTP. Five patients reached the operating room (OR) in a hemodynamically stable condition. Five patients underwent bilateral salpingectomy (5/7, 71.4%) in spite of the early preoperative diagnosis. Bilateral tubal rupture was present in one case, while in three, this surgery was done for the pathologic condition of the tubes and electively in one case upon the request of the patient. Unilateral salpingectomy was performed in the remaining two patients. Preoperative diagnosis could have averted catastrophic hemorrhage but did not prevent the need to perform bilateral salpingectomy and hence did not provide a safeguard to the integrity of the tubes. Five of these seven cases had no identifiable risk factor and two were primigravidas.
TVUS was reportedly available in 53/59 cases. After excluding the eight cases with asynchronous BTP, 45 cases were analyzed. Seven cases 7/45 (15.5%) were diagnosed with BTP preoperatively. In 7/45 cases, no diagnosis of any ectopic was reached (15.5%), while in 31/45 cases (68.8%), suspicion of unilateral tubal EP was made. Of these, 25 cases had unilateral ectopic mass while in six, a unilateral ectopic gestational sac was visualized.
Pathogenesis
Several theories have been postulated regarding the genesis of spontaneous EP. These include damage to the endosalpinx, distortion of pelvic anatomy, Mullerian malformations and hormonal imbalance during the menstrual cycle and late ovulations [44]. For a bilateral EP to occur, in addition to the aforementioned factors, polyovulation should take place. There might be no single theory to explain the genesis of all cases with BTP. The origin of the two mature ova responsible for BTP is not well defined and whether they originate from one or both ovaries is also not clear. This can be clarified only upon examining the laterality of corpus of luteum of pregnancy (CLP) in natural conceptions. Unfortunately, documentation and description of ultrasound features in almost all case reports has been poor. Bilateral CLP was described in one case, favoring the occurrence of BTP where each ovum originated from the adjacent ovary [20]. The other possibility is upon finding only one CLP, suggesting that the two ova originated from a single ovary with subsequent implantation of one ovum in the adjacent tube and a trans-peritoneal migration of the second fertilized ovum to implant in the contralateral tube. This theory of transperitoneal migration was advanced by Tabichnikoff to explain the discrepancy in size between both EPs [45]. The theory of transperitoneal migration as the only pathogenesis of EP has been entirely challenged by Zeil and co-workers who favored different mechanism for EP [46]. Poly-ovulation is an essential requirement for twinning to occur and this can manifest as heterotopic pregnancies where one fetus is intrauterine and one EP in each tube [36, 47], twin ectopics in one tube [48] and twin ectopics: one in each tube as in cases with BTP. Although twinning is not uncommon among humans, abnormal ectopic twinning is extremely rare. Interestingly, BTP was found by Lopez et al to be the rarest form of abnormal twinning types [49]. Twinning proneness was identified long ago by Onuigbo et al to be a potential cause of BTP [41]. Similar observations were made by Makinde et al who reported that the incidence of BTP in native African population is estimated to be 1 in 51 EPs possibly due to higher rate of twinning and untreated pelvic inflammatory disease (PID) [50]. Whether populations prone to twinning (Africans) are really at increased risk of developing BTP or twin ectopics in a single tube or heterotopic pregnancies as suggested by Onuigbo et al is an interesting remark that deserves exploration.
There is a substantiated doubt that some of the BTP could be a combination of old unresorbed (chronic) EP and a new fresh EP in the contralateral tube as was reported by Xiromeritis et al in 2015 [51]. In this series, several cases were found to have apparent discrepancy in the size of ectopics. Four reports even described the presence of mummified fetal parts in one of the tubes but not the other [26, 36, 52, 53]. On several instances, there was a clear history of previous ectopic but in others this was not even established. Xiromeritis et al suggested that evaluation of tubal patency should be done after medically treated EP, especially that chronic EP can be subtle and not producing beta-hCG [51]. Sequential ovulation as suggested by Kobayashi et al is another plausible explanation for the size discrepancy that was recognized in some cases of BTP [54]. He suggested that the second tubal pregnancy occurred after the demise of the first, whereas if the first EP has spontaneously aborted or was in the process of aborting, a second ovulation might have occurred during this time period, resulting in the occurrence of BTP. He reported a case, where an intrauterine pregnancy occurred during an incomplete abortion of an extra-uterine gestation [54]. But for this to occur, there must be normal uninterrupted intercourse. Sequential ovulation can explain some cases of non-simultaneous spontaneous BTP if sexual life was not interrupted for any reason. But in the absence of intercourse in the interval between the two diagnoses, pregnancy cannot materialize even if ovulation recurred.
In an effort to explain asynchronous or non-simultaneous cases of BTP where a time-lag was observed between the diagnosis of the two EPs, Tabichnikoff et al suggested the theory of superfetation, which implies that fertilization and development of a second oocyte occurred when a woman is already pregnant [45]. This, though, is considered to be an extremely rare event in human beings. In fact, this was impossible to exist in cases where patients denied any intercourse in the time interval separating the occurrence of the two EPs. The theory of superfetation was totally rejected by Edelstein et al [3], who were in favor of the theory of growth-potential difference among EPs suggested earlier by Poland in1976 [55].
Actually, BTP could better be comprehended if we understand the natural history and the conduct of individual EPs. It is well-known that tubal EP grows according to its own programming. Rapid, slow, very slow and even arrested growths are all well-recognized patterns of growth in EPs. If the pace of growth is similar for both EPs, we would identify BTP concurrently and simultaneously; however, given their different growth-pace potential, if one EP continued to progress while the second one stopped growing, this would result in discrepancy in size. This is exactly what happens when EP transforms into chronic ectopic. These can persist asymptomatic for a while and even might not produce enough beta-hCG. This different potential of growth can explain also the non-simultaneous appearance of the two EPs. Discrepancy in the size was observed in 22/59 cases in this series. In four cases, one tube contained a conceptus of few millimeters size while the second EP enclosed visible fetal parts. The only reliable methodology to rule out the presence of chronic EP has been performing hysterosalpingography (HSG) to ensure tubal patency especially when following medical treatment or conservative tubal surgery for EP. Walter et al described a case BTP where one was acute EP and the second was chronic EP following failed medical treatment of previous EP [56].
Surgical findings
Laparoscopy was used in 21/59 (35.6%) for surgery while laparotomy was used in 27/59 cases. The ectopic location in first tube was in the ampulla in 24 cases, in the isthmus in 13, while no mention of the location in 13. The location in the second tube was ampullary in 33 cases, isthmic in six and no mention in 11. The EP in the first tube was found to be ruptured in 25 cases and in the second tube in four cases. Bilateral rupture was encountered in one case only.
Management guidelines
Two clinical guidelines for the management of EP have been issued by the American College of Obstetrics and Gynecology and the Royal College of Obstetricians and Gynecologist without any reference to BTP, probably reflecting the rarity of this clinical condition [57, 58]. Furthermore, except for case reports or series, we could find no studies dedicated for the management of BTP. In spite of the 10% risk of recurrence following conservative surgery, this did not deter gynecologists from performing salpingostomy to treat ETP. This even became the standard of care especially when coupled with another minimally invasive procedure, i.e., laparoscopy. These, however, could be done only in stable patients before extensive damage of the tube or hemodynamic instability. Early diagnosis could be successfully materialized in unilateral EP with the advent of TVUS and the wide-spread use of beta-hCG kits, but, unfortunately, not in BTP where diagnosis is still made intraoperative in the vast majority of cases.
The choice of treatment in general depends, as with unilateral EP, on the condition of the patient, extent of tubal damage and the desire for future fertility [26], in addition to the size and location of the ectopic, and the level of beta-hCG. Treatment options for cases with spontaneous BTP are basically the same as with unilateral EP. The choice of surgical treatment might differ between spontaneous BTP and those following ART. Conversely, there is a tendency to perform radical surgery in the form of bilateral salpingectomy among patients with infertility using ART even when contralateral tubes seem healthy; however, in spontaneous BTP, the trend is to do salpingostomy if the tube looks benign. In fact, this has shown to result in the only successful pregnancy in this series [59]. Two similar cases were traced in the literature with history of BTP treated with salpingectomy on one and salpingostomy on the second tube, and were reported to have had successful subsequent pregnancies [60]. Likewise, the choice of minimally invasive interventions can be implemented only if diagnosis was achieved early, which currently might not be possible in BTP unless substantial improvement in early diagnosis materialize. BTP will remain in the vast majority of cases an intraoperative diagnosis. This might prevent future development of BTP when a patient is destined to rely on ART, which itself is known to increase the risks of BTP. On the other hand, for spontaneous BTP, the general principles of management of EP are followed. This entails performing laparoscopic conservative surgery whenever possible [61]. When the hemodynamic status of the patient is stable with intact BTP, a medical treatment with methotrexate could be instituted. In 1997, a case of failed treatment with single dose IM methotrexate was reported by Marcovici et al before discovering BTP at laparotomy. They questioned whether the use of the recommended standard single dose of 50 mg/kg methotrexate for unilateral EP is valid for BTP [42]. In 2001, Mock et al reported successful use of methotrexate injection under ultrasound guidance in a case of BTP following the use of ART [62]. Five cases in this group were subjected to this modality of treatment [9, 11, 43, 63, 64]. It was successful in one case of 8 weeks + 5 days gestation where methotrexate was initiated when prenatal diagnosis of undisturbed unilateral EP was made, then another EP was diagnosed 1 week later upon close follow-up with beta-hCG and TVUS. Methotrexate treatment was extended and was successful [9]. Another successful case at 6 weeks was reported by Ghosh et al where the diagnosis of the second EP was made several days postoperatively and since the patient was stable, they opted for medical treatment [64]. In these two cases, no pretreatment diagnosis of BTP was made. Medical treatment failed in the remaining three cases. Given this small number of cases we cannot arrive at solid conclusions concerning the appropriateness of this treatment modality. We observed no relation between the level of beta-hCG and the success of treatment in these five cases. The levels among failed treatment cases were 534, 1,866 and 13,073 IU/L, while among responders it was 2,082 and 2,512 IU/L. Other important details like the size of the ectopic sacs/masses or the presence of viable fetuses were not reported in all these cases. Should there be more stringent conditions to select whom can receive this treatment or should larger doses of methotrexate be used according to a different protocol (other than the single dose) is not clear at this point. It is worth mentioning that only one case of failed treatment (1/3) ended with bilateral salpingectomy [43]. Laparoscopic surgical treatment is preferred to open procedures, because the patient recovers more quickly and subsequent rates of intrauterine and EP are similar [65]. The possibility of a uterine pregnancy in a patient with unruptured tubal EP is around 24-60% [66].
Failure to inspect the contralateral tube, ovaries and the whole pelvis even in the presence of dense adhesions was responsible for failure to identify BTP during surgery in several cases. Typically, these patients presented few days later with worsening symptoms and increasing levels of beta-hCG and at times with acute abdomen due to ruptured EP in the contralateral tube.
Future fertility potential
It is a well-known fact that fertility rate is negatively affected following previous EP and may even worsen with a history of preexisting infertility [20]. Likewise, BTP is not an exception. Recurrence rate of unilateral EP is estimated in the range of 6-16%, while recurrence after BTP is hard to figure owing to the rarity of this condition, nonetheless, it is definitely increased following BTP [67]. No information was available in this series concerning recurrence rate of BTP, though a history of previous unilateral EP was positive in four cases and history of infertility was present in six cases.
The type of surgery, laparotomy vs. laparoscopy or salpingectomy vs. salpingostomy was found to exert no influence on the success of future conception. Sommer et al in 2002 reported that most cases with BTP were managed surgically with bilateral salpingectomy thus giving a gloom perspective of the outcome in BTP [68]. Actually, salpingectomy is commonly done after thorough evaluation of the condition of the tube, the contralateral tube, the patient’s plans for future fertility and the hemodynamic status of the patients. In this series of 59 patients, 21 (33.6%) underwent bilateral salpingectomy. This does not reflect the actual frequency of bilateral ruptured ectopics. In fact, 29 cases had their first EP ruptured; yet, salpingectomy was performed on 43 patients. The second ectopic was ruptured in five cases only but again 24 salpingectomies were done for the second tube reflecting the fact that causes other than ruptured ectopic were determinant in the decision of this surgery. Bilateral tubal rupture was found only in one case; however, 21 bilateral salpingectomies were done. When compared to the 100% salpingectomy rate reported by Sommer et al, the 40% observed in this review is an interesting finding, probably reflecting earlier identification before rupture of the EP and possibly less existing reasons to remove completely both tubes [68].
Successful term pregnancy was reportedly achieved in one case when BTP was treated with salpingectomy for one tube and salpingostomy for the other tube [59]. A similar case was also reported by Mathelier [60].
Suggested key points to improve management
It is extremely important to inspect the entire pelvis, including the contralateral tube, during surgery performed for unilateral EP, even if this required extensive lysis of adhesions.
HSG is recommended for patients who were treated medically (methotrexate) or with conservative surgery, in order to explore the patency of the tube, as the fate of EP might not always be complete resolution but might rather end with chronic EP. These might not produce high levels of beta-hCG and are hence not detectable with serial beta-hCG surveillance.
Tubal surgery has long been found to be an important risk factor for the development of EP [29]. Likewise, simple transection of the tubes (Pomeroy procedure) has also been shown to be complicated with BTP in two cases in our series and in other case series [39, 69, 70]. This method of sterilization has long been criticized by Levy et al in 1988 after reporting the first case of BTP following this procedure, and the same may well be true for clip-sterilization [71, 72]. Partial salpingectomy for the treatment of EP should be critically reassessed and possibly replaced with complete salpingectomy in the surgical treatment of EP. Tubal remnants pose a risk for recurrent EP [73, 74].
Conclusion
Early identification of BTP is crucial to minimize its related devastating complications. This requires some reforms in our clinical practice. Patients are advised to report pregnancy as early as possible in order to verify the location of pregnancy. This should not be limited to high-risk groups, as around 50% of patients with BTP lack such risk factors. Furthermore, in early pregnancy, routine meticulous sonographic examination of the whole pelvis should be executed for intra-, as well as, extrauterine pregnancies. Gynecologists should explore contralateral tube and consider performing complete salpingectomy while performing surgery on ETP. Furthermore, “Pomeroy technique” used for BTL is better replaced with bilateral salpingectomy. Close follow-up with clinical, sonographical and serial serum beta-hCG should be routinely implemented for all ETP even following salpingectomy.
| Supplementary Material | ▴Top |
Suppl 1. Relevant Clinical, Diagnostic and Surgical Features of the Case Reports of the Review.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained according to the guidelines.
Author Contributions
All authors analyzed the diagnosis and the management of the case, and they edited and approved the last version of the manuscript. MK and MKR did the literature review.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Centers for Disease Control and Prevention (CDC). Ectopic pregnancy - United States, 1990-1992. MMWR Morb Mortal Wkly Rep. 1995;44(3):46-48.
- Bouyer J, Coste J, Fernandez H, Pouly JL, Job-Spira N. Sites of ectopic pregnancy: a 10 year population-based study of 1800 cases. Hum Reprod. 2002;17(12):3224-3230.
doi pubmed - Edelstein MC, Morgan MA. Bilateral simultaneous tubal pregnancy: case report and review of the literature. Obstet Gynecol Surv. 1989;44(4):250-252.
doi - Abrams RA, Kanter AE. Bilateral simultaneous extrauterine pregnancy. Am J Obstet Gynecol. 1948;56(6):1198-1200.
doi - Coulier B, Malbecq S, Brinon PE, Ramboux A. MDCT diagnosis of ruptured tubal pregnancy with massive hemoperitoneum. Emerg Radiol. 2008;15(3):179-182.
doi pubmed - Bustos Lopez HH, Rojas-Poceros G, Barron Vallejo J, Cintora Zamudio S, Kably Ambe A, Valle RF. [Conservative laparoscopic treatment of bilateral ectopic pregnancy. 2 case reports and review of the literature]. Ginecol Obstet Mex. 1998;66:13-17.
- De Los Rios JF, Castaneda JD, Miryam A. Bilateral ectopic pregnancy. J Minim Invasive Gynecol. 2007;14(4):419-427.
doi pubmed - El Hakim E, Cahill D. Concurrent bilateral ectopic pregnancy after recurrent miscarriages in a fertile woman. J Obstet Gynaecol. 2009;29(4):359.
doi pubmed - Savant R, Peacock S, Piskorowskyj N. Spontaneous bilateral ectopic pregnancy: case report: P2. 49. Br J Obstet Gynaecol. 2012;119.
- Li W, Wang G, Lin T, Sun W. Misdiagnosis of bilateral tubal pregnancy: a case report. J Med Case Rep. 2014;8:342.
doi pubmed - Seol HJ, Tong SY. Spontaneous bilateral tubal pregnancy following hysterosalpingography. Arch Gynecol Obstet. 2014;289(5):923-924.
doi pubmed - Gardyszewska A, Dobrowolska-Redo A, Czajkowski K, Smolarczyk R, Roszkowski P. Non-simultaneous two-stage detection of spontaneous bilateral isthmic tubal pregnancy. Ginekol Pol. 2016;87(10):728.
doi pubmed - Memon F, Matar M. Spontaneous bilateral tubal pregnancies: A two staged presentation. Journal of Cases in Obstetrics and Gynecology. 2016;3(3):77-79.
- Brown NE, Singer SA, Suyama J. Delayed Detection of Spontaneous Bilateral Tubal Ectopic Pregnancies After Methotrexate Treatment. J Emerg Med. 2017;53(4):563-567.
doi pubmed - Haddad A, Ho D, Campbell D. Spontaneous synchronous bilateral ectopic pregnancy. Journal of Gynecologic Surgery. 2017; 33(5):202-204.
doi - Zhu B, Xu GF, Liu YF, Qu F, Yao WM, Zhu YM, Gao HJ, et al. Heterochronic bilateral ectopic pregnancy after ovulation induction. J Zhejiang Univ Sci B. 2014;15(8):750-755.
doi pubmed - Gerli S, Giordano C, Di Renzo GC. Tubal pregnancy after clomiphene treatment: are you sure is not bilateral? Gynecol Endocrinol. 2016;32(8):607-608.
doi pubmed - Fishback HR. Bilateral simultaneous tubal pregnancy. American Journal of Obstetrics and Gynecology. 1939;37:1035-1037.
doi - Shetty JP, Shetty B, MakkanaVar JH. A rare case of bilateral tubal pregnancy. Journal of Indian Medical Association. 2011;109(7):5-7.
- Eze JN, Obuna JA, Ejikeme BN. Bilateral tubal ectopic pregnancies: a report of two cases. Ann Afr Med. 2012;11(2):112-115.
doi pubmed - Sheeba M, Supriya G. Spontaneous bilateral tubal gestation: a rare case report. Case Rep Obstet Gynecol. 2016;2016:8526903.
doi pubmed - Ahuja M, Mehta A, Goel N, Jamal S. An increasing trend of bilateral tubal ectopic gestation reported over the past two decades: a case report and review of literature. International Journal of Reproduction, Contraception Obstetrics and Gynecology. 2018;7(6):2518-2520.
doi - Campo S, Campo V, Gambadauro P. Bilateral tubal pregnancy following in vitro fertilization and embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2003;110(2):237-239.
doi - Issat T, Grzybowski W, Jakimiuk AJ. Bilateral ectopic tubal pregnancy, following in vitro fertilisation (IVF). Folia Histochem Cytobiol. 2009;47(5):S147-148.
doi pubmed - Barnhart KT, Sammel MD, Gracia CR, Chittams J, Hummel AC, Shaunik A. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86(1):36-43.
doi pubmed - Andrews J, Farrell S, Andrews J. Spontaneous bilateral tubal pregnancies: a case report. J Obstet Gynaecol Can. 2008;30(1):51-54.
doi - Marcus SF, Brinsden PR. Analysis of the incidence and risk factors associated with ectopic pregnancy following in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10(1):199-203.
doi pubmed - Makinde OO, Ogunniyi SO. Bilateral tubal and twin pregnancies in Ile-Ife, Nigeria. Int J Gynaecol Obstet. 1990;33(4):365-367.
doi - Ankum WM, Mol BW, Van der Veen F, Bossuyt PM. Risk factors for ectopic pregnancy: a meta-analysis. Fertil Steril. 1996;65(6):1093-1099.
doi - Lobo RA, Patricio L, Milheiras E, Cordeiro A, Hermida M. Case Report/Caso Clinico. Acta Obstet Ginecol Port. 2012; 6(3):141-144.
- Xu H. A spontaneous bilateral tubal pregnancy: A case report. Medicine (Baltimore). 2018;97(38):e12365.
doi pubmed - Martinez J, Cabistany AC, Gonzalez M, Gil O, Farrer M, Romero JA. Bilateral simultaneous ectopic pregnancy. South Med J. 2009;102(10):1055-1057.
doi pubmed - Grechukhina O, English DP, Hong W, Kaza R, Ratner E. Spontaneous ruptured heterotopic fallopian tube pregnancy: a challenging case. Int J Womens Health Wellness. 2015.
doi - Nacharaju M, Vellanki VS, Gillellamudi SB, Kotha VK, Alluri A. A rare case of chronic ectopic pregnancy presenting as large hematosalpinx. Clin Med Insights Reprod Health. 2014;8:1-4.
doi pubmed - Ryan MT, Saldana B. Bilateral tubal ectopic pregnancy: a tale of caution. Acad Emerg Med. 2000;7(10):1160-1163.
doi pubmed - Vyas NM, Manjeera L, Shetty D, Rai S. Spontaneous bilateral simultaneous tubal pregnancy. Nepal Journal of Obstetrics and Gynaecology. 2013;8(1):41-42.
doi - Gamberdella FR, Marrs RP. Heterotopic pregnancy associated with assisted reproductive technology. Am J Obstet Gynecol. 1989;160(6):1520-1522; discussion 1522-1524.
doi - Pramanick A, Peedicayil A, Shah A. Bilateral tubal pregnancy with intrauterine pregnancy in a natural conception cycle along with liver cell failure: case report and review of literature. J Obstet Gynaecol India. 2014;64(Suppl 1):50-52.
doi pubmed - Mandal RD, Ghosh S, Mitra S, Basak A, Naskar P, Seth K, Halder A. Bilateral tubal pregnancy: A diagnostic dilemma. Open Journal of Obstetrics and Gynecology. 2013;3(09):639.
doi - Norris S. Bilateral simultaneous ectopic pregnancy. Canadian Medical Association Journal. 1953;68:379-381.
- Onuigbo WI. Twin proneness among Igbo women attending a self-referral out-patient clinic in Enugu. International Journal of Medicine and Health Development. 2007;12(1):1-4.
- Marcovici I, Scoccia B. Spontaneous bilateral tubal ectopic pregnancy and failed methotrexate therapy: a case report. Am J Obstet Gynecol. 1997;177(6):1545-1546.
doi - Wali AS, Khan RS. Spontaneous bilateral tubal pregnancy. J Coll Physicians Surg Pak. 2012;22(2):118-119.
- Atrash HK, Friede A, Hogue CJ. Ectopic pregnancy mortality in the United States, 1970-1983. Obstet Gynecol. 1987;70(6):817-822.
- Tabachnikoff RM, Dada MO, Woods RJ, Rohere D, Myers CP. Bilateral tubal pregnancy. A report of an unusual case. J Reprod Med. 1998;43(8):707-709.
- Ziel HK, Paulson RJ. Contralateral corpus luteum in ectopic pregnancy: what does it tell us about ovum pickup? Fertil Steril. 2002;77(4):850-851.
doi - Selim MF, Abdou MM, Al-Bahrani G. Bilateral simultaneous tubal ectopic pregnancy with a third intrauterine pregnancy: a rare case of heterotopic pregnancy. Journal of Gynecologic Surgery. 2018; 34(6):301-303.
doi - Urunsak I, Kadayifci O, Atay Y, Demir S, Guzel AB. A unilateral tubal twin pregnancy. Ann Saudi Med. 2006;26(4):333.
doi pubmed - Fox EJ, Mevs FF. Simultaneous bilateral tubal pregnancies. Report of 2 cases. Obstet Gynecol. 1963;21:499-501.
- Makinde OO, Ogunniyi SO. Ectopic pregnancy in a defined Nigerian population. Int J Gynaecol Obstet. 1990;33(3):239-241.
doi - Xiromeritis P, Margioula-Siarkou C, Miliaras D, Kalogiannidis I. Laparoscopic excision of coexisting left tubal and right pseudotubal pregnancy after conservative management of previous ectopic pregnancy with methotrexate: an unusual clinical entity. Case Rep Surg. 2015;2015:645826.
doi pubmed - Amine BH, Haythem S. Extra-uterine twin pregnancy: case report of spontaneous bilateral tubal ectopic pregnancy. Pan Afr Med J. 2015;20:435.
doi - Sinha P, Koumousidis A, Carr M, Sofoudis C. Ultrasound diagnosis of bilateral ectopic pregnancy: Still a myth? Hellenic Journal of Surgery. 2015;87(6):490-492.
doi - Kobayashi F, Sagawa N, Konishi I, Tsuruta Y, Fujiwara H, Mori F. Spontaneous conception and intrauterine pregnancy in a symptomatic missed abortion of ectopic pregnancy conceived in the previous cycle. Hum Reprod. 1996;11(6):1347-1349.
doi pubmed - Poland BJ, Dill FJ, Styblo C. Embryonic development in ectopic human pregnancy. Teratology. 1976;14(3):315-321.
doi pubmed - Walter JE, Buckett WM. Spontaneous bilateral chronic and acute tubal ectopic pregnancies following methotrexate treatment. Aust N Z J Obstet Gynaecol. 2004;44(3):267.
doi pubmed - Barnhart MSCE, Franasiak JM. ACOG Practice Bulletin No. 191: tubal ectopic pregnancy. Obstetrics Gynecology. 2018;131(2):409-411.
doi pubmed - Kelly AJ, Sowter MC, Trinder J. The management of tubal pregnancy. London: RCOG Press; 2004.
- Rani VRS, Puliyath G. Viable intrauterine pregnancy after spontaneous bilateral tubal ectopics in a multiparous woman: a case report. J Med Case Rep. 2013;7:159.
doi pubmed - Mathelier AC. Fertility after bilateral tubal pregnancy - modern treatment considerations. Int J Fertil. 1990;35(3):160-163.
- Seeber BE, Sammel MD, Guo W, Zhou L, Hummel A, Barnhart KT. Application of redefined human chorionic gonadotropin curves for the diagnosis of women at risk for ectopic pregnancy. Fertil Steril. 2006;86(2):454-459.
doi pubmed - Mock P, Olivennes F, Doumerc S, Frydman R, Fernandez H. Simultaneous bilateral tubal pregnancy after intracytoplasmic sperm injection treated by conservative medical treatment. Interest of sonographic follow-up. Eur J Obstet Gynecol Reprod Biol. 2001;94(1):155-157.
doi - Othman M, Karali S, Badawi K, Mossa H. Bilateral ectopic pregnancy: case report. Webmed Central Obstetrics and Gynaecology. 2013;4(7):WMC004354.
- Ghosh A, Borlase D, Ajala T, Kelly AJ, Ibrahim Z. Dilemmas in management of bilateral ectopic pregnancies - report of two cases and a review of current practice. Gynecological Surgery. 2014;11(3):191-196.
doi - Hajenius PJ, Mol F, Mol BW, Bossuyt PM, Ankum WM, van der Veen F. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;1:CD000324.
doi pubmed - al-Awwad MM, al Daham N, Eseet JS. Spontaneous unruptured bilateral ectopic pregnancy: conservative tubal surgery. Obstet Gynecol Surv. 1999;54(9):543-544.
doi pubmed - Hoffmann S, Abele H, Bachmann C. Spontaneous bilateral tubal ectopic pregnancy: incidental finding during laparoscopy - brief report and review of literature. Geburtshilfe Frauenheilkd. 2016;76(4):413-416.
doi pubmed - Sommer EM, Reisenberger K, Bogner G, Nagele F. Laparoscopic management of an unrecognized spontaneous bilateral tubal pregnancy. Acta Obstet Gynecol Scand. 2002;81(4):366-368.
doi pubmed - Reddy A, Chowdhary S, Saxena R, Venkatesh S, Pandey P. Spontaneous bilateral ectopic pregnancy. Br J Obstet Gynaecol. 2012;119.
- Shah JP, Parulekar SV, Hinduja IN. Ectopic pregnancy after tubal sterilization. J Postgrad Med. 1991;37(1):17-20.
- Levy JS, Rosenzweig BA, Blumenthal L. Bilateral tubal pregnancies after tubal sterilization. Obstet Gynecol. 1988;72(3 Pt 2):494-495.
- Kauppi-Sahla M, Rintala H, Makinen J. Bilateral tubal pregnancy: a case report and review of the literature. Eur J Obstet Gynecol Reprod Biol. 1991;40(2):145-147.
doi - Chou LL, Huang MC. Recurrent ectopic pregnancy after ipsilateral segmental salpingectomy. Taiwan J Obstet Gynecol. 2008;47(2):203-205.
doi - Muppala H, Davies J. Spontaneous proximal tubal stump pregnancy following partial salpingectomy. J Obstet Gynaecol. 2009;29(1):69-70.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
