| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Original Article
Volume 9, Number 4, December 2020, pages 79-95
The Association Between Pelvic Organ Prolapse, Pelvic Pain and Pelvic Reconstructive Surgery Using Transvaginal Mesh: A Secondary Analysis of a Prospective Multicenter Observational Cohort Trial
Bernhard Liedla, g, Klaus Goeschenb, Naira Grigoryanc, Suzette E. Sutherlandd, Alexander Yassouridise, Magdalena Witczaka, Jan-Paul Rooversf
aZentrum fur Rekonstruktive Urogenitalchirurgie, Urologische Klinik Planegg, Germany
bMedical School of Hannover, Neustadt/Weinstrasse, Germany
cGynecology, Beckenbodenzentrum Planegg, Germany
dUrology, The Pelvic Health Center, University of Washington, Seattle, WA, USA
eEthical Committee, Ludwig-Maximilians-University Munich, Germany
fAcademic Medical Centre/University of Amsterdam, Amsterdam, The Netherlands
gCorresponding Author: Bernhard Liedl, Zentrum fur Rekonstruktive Urogenitalchirurgie, Urologische Klinik Planegg, Germeringer Strasse 32, D-82152 Munchen-Planegg, Germany
Manuscript submitted September 29, 2020, accepted November 11, 2020, published online December 15, 2020
Short title: POP and Pelvic Floor Reconstruction
doi: https://doi.org/10.14740/jcgo696
| Abstract | ▴Top |
Background: The aims of the study were to examine the type, severity and prevalence of pain in women with pelvic organ prolapse (POP) before and after pelvic floor reconstructive surgery, and to evaluate the effect of POP reconstruction over a period of 2 years.
Methods: The study data were collected in a past multicenter prospective study (Propel-Study; Clinical Trials.Cov. Identifier: NCT00638235), where a total of 281 women with stage 2-4 symptomatic POP underwent prolapse repair using the transvaginal, single-incision “Elevate” technique for anterior/apical and posterior/apical prolapse. The degree of POP repair during a follow-up of 2 years was the primary endpoint of the study and has already evaluated previously. In the Propel trial subjective assessments of presumable POP symptoms before and 6, 12 and 24 months after surgery were registered too utilizing the pelvic floor disorder inventory (PFDI) questionnaire. The investigation of all or specific domains of PFDI symptoms were declared as secondary objectives of that study. The present treatise is concentrated on the evaluation of the PFDI pain symptoms consisting of six questions for describing different types of pain.
Results: Preoperatively, 67% of all POP patients reported moderate-to-severe pain, mainly of visceral character. Pelvic floor reconstructive surgery resulted in significant cure or improvement in all pain types, intensities and locations, and these improvements were stable over 2 years. The largest reduction in pain symptoms following POP repair was registered for visceral complaints (87%), followed by the anterior (84%) and then the posterior (45%). Patients with cystocele had significantly more pain pre- and postoperatively than those with enterocele/rectocele. The best cure effects overall were achieved in cystocele patients with visceral or anterior pain (> 96%). No correlation was found between POP stage and pain intensity preoperatively. The best pain cure rates after surgery were obtained in patients with pronounced POP and pain in the visceral and anterior areas (> 93%). Surgically induced de novo pain improved with time from 16% (6 months) to 11% (12 months) and then 7% (24 months).
Conclusion: POP of stage 2 and greater can be associated with moderate-to-severe pain, which can be improved and cured by mesh-supported prolapse repair. Postoperative de novo pain does occur, although its incidence and severity appear to be reduced over time.
Keywords: Chronic pelvic pain; Pelvic organ prolapse; Posterior fornix syndrome; Pelvic floor reconstruction; Pelvic mesh
| Introduction | ▴Top |
The use of alloplastic materials in pelvic floor reconstruction can produce de novo pain [1-4]. Reports of adverse events including pain and missing evidence that the probable benefit of the mesh devices outweighs their probable risks influenced the Federal Drug Administration (FDA) to order that all manufacturers in the United States had to stop selling and distributing surgical meshes for transvaginal repair of anterior compartment prolapse in April 2019 [4]. Introduction of microporous light-weight mesh should reduce tissue reaction and infection rate and presumably pain [5, 6]. It has been suggested that meshes should be avoided in women with pre-existing chronic pain syndromes [7].
On the other hand, pelvic pain is reported to be caused by pelvic organ prolapse (POP) and eventually be cured by adequate pelvic floor surgery [8, 9].
In 1938, Heinrich Martius stated that in approximately 30% of cases, backaches were attributed to organic factors provoked by damaged suspensory or supportive ligaments of the pelvic organs [10, 11].
In 1993, Petros and Ulmsten described chronic pelvic pain syndrome (CPPS) as being caused by lax uterosacral ligaments as part of the “posterior fornix syndrome” [12], along with other pelvic symptoms such as nocturia, urgency and abnormal emptying. They reported a significant cure rate of CPPS and other posterior fornix symptoms following repair of the uterosacral ligaments and vaginal apical reconstruction [12].
In an extensive review in 2015, Goeschen [8] explained how sympathetic (T12-L2), parasympathetic (S2-S4) and somatic nerves (S2-S4) could be stretched in women with POP and cause different pain qualities and localizations, from lower abdominal and posterior area pain to pelvic pain and vulvodynia.
Despite these publications, definitions of and numerous review papers on chronic pelvic pain (CPP) and bladder pain syndrome (BPS) either fail to mention [13-23] or mention purely peripherally [24] the possible causal relationship between POP and CPP. And in none of these reviews is surgical POP repair identified as a plausible treatment option.
CPP affects many women, and it has been debated to date on whether the existence of POP is an independent risk factor for CPP. In 2004, Williams et al [25] reviewed the existing literature using “chronic pelvic pain” as a keyword and selected articles focusing on the duration, location and definition of CPP. Disappointingly, they found a number of notable omissions within these publications: 93% did not specify the location of pain, 44% did not specify the duration, 74% did not address etiology or pathology in their definitions and 95% did not consider comorbidities.
Meanwhile, there is much more information today about CPP [8, 24]. But even today, numerous CPP conditions are still deemed to be of unknown origin. Due to the numerous possible etiologies for pelvic pain, the definition of CPP is still incongruent and ambiguous in the literature. An extract of the learned society definitions can be summarized as follows: 1) CPP is a chronic or persistent type of pain perceived in structures related to the pelvis [24]; 2) CPP is pain in the pelvic area that lasts for 6 months or longer [26]; 3) CPPS is the occurrence of CPP when there is no proven infection or other obvious local pathology that may account for the pain. CPPS is often associated with negative cognitive, behavioral, sexual or emotional consequences, as well as with symptoms suggestive of lower urinary tract, sexual, bowel or gynecological dysfunction [27, 28].
Bearing these controversies in mind, we were prompted to further analyze the data of the Propel study to investigate the interrelationship between POP and pelvic pain before and after surgical POP reconstruction, using the following hypotheses: 1) Women with a POP-defect greater than stage 1 reveal in a considerable proportion pelvic pain symptoms too, which may show some significant differences in their severity when comparing specific subgroups of the sample population; 2) Pelvic pain symptoms can be localized in specific pelvic regions showing characteristic severity profiles both in the total sample population and in some specific subgroups of it; 3) POP repair can significantly remedy type-dependent pain severity for a long time.
The profiles of pain severity from baseline to 2 years follow-up show considerable differences with respect to type of pain complaint as well to position and stage of POP.
Furthermore explorative interest of the study was directed to the incidence of de novo pain following mesh-supported POP repair, to the pain severity transitions between pre- and postoperative phase and to the coexistence of other pelvic floor symptoms as well.
| Materials and Methods | ▴Top |
The Propel study was conducted from 2008 to 2012 by 16 centers in the USA and Europe (urology, urogynecology and gynecology sites proficient in prolapse repair and transvaginal mesh use) to primarily assess the effect of the Elevate single-incision, transvaginal prolapse reconstruction systems (American Medical Systems, Minnetonka, MN, USA) on the anatomical correction of anterior/apical or posterior/apical prolapse stage ≥ 2, with anatomical success being defined as POP ≤ stage 1. Institutional review board (IRB) approval was obtained by all centers. This study was conducted in compliance with the ethical standards of the responsible institutions on human subjects as well as with the Helsinki Declaration.
The various outcome measures, the inclusion and exclusion criteria are reported in the ClinicalTrials registration [29].
Secondary endpoints included a variety of quality of life (QOL) measures through utilization of the pelvic floor distress inventory (PFDI) questionnaire at baseline and 6, 12 and 24 months postoperatively. This observational study was done to evaluate the effect of POP reconstruction specifically on pain symptoms.
In the Propel study, a total of 281 female patients with POP (≥ stage 2) and a mean age of 63.2 ± 10.6 years were included in the Propel study. Four patients with incomplete data in the PFDI questionnaire were excluded. This study therefore concentrated on 277 patients with anterior/apical POP (n = 142) and posterior/apical POP (n = 135). According to the POP quantification system (POP-Q), 122 patients had stage 2 POP, and 150 had stage 3 or 4 POP.
Pain-related symptoms were identified by PFDI questions 1 (pressure in the lower abdomen), 2 (pain in the lower abdomen or genital area), 3 (heaviness or dullness in the pelvic area), 6 (pelvic discomfort when standing or upon physical exertion), 7 (pain in the lower posterior area most days) and 46 (abdominal or lower posterior area pain when straining for any reason). The six types of pain symptoms could be well assigned to three complaint areas: “anterior area” (PFDI 1, 2), “visceral area” (PFDI 3, 6) and “posterior area” (PFDI 7, 46). On the PFDI, subjective answers and corresponding scoring related to these pain symptoms with respect to degree of bother include: “no symptoms” = score 0, “yes/not at all bothersome” = score 1, “somewhat bothersome” = score 2, “moderately bothersome” = score 3, or “quite a bit bothersome” = score 4. Values were obtained at baseline, then postoperatively at 6, 12 and 24 months. The hypotheses of the study were predominantly focused on the “moderately or quite a bit” bother of the symptoms or equivalently on symptom-intensity scores ≥ 3.
Statistical analyses
Given the categorical data structure of the investigated pain symptoms, statistical evaluation was mainly based on frequency analyses related to single or combined (compound) symptom outcomes. To the later belongs the compound outcome “R2” that corresponds to a “moderately or quite a bit” bothersome symptom assessment, and its pedant “R1” that corresponds to a “no symptoms or yes/not at all or somewhat” bothersome symptom assessment.
Towards the pain location regions, statistical analysis was additionally performed on two specific pain events defined as follows: “irrelevant pain complaints” (IPCs), when both symptoms of the corresponding region were evaluated in terms of their severity in patients with R1, and “relevant pain complaints” (RPCs), when at least one of the two symptoms were evaluated in patients with R2. By considering the “complete pelvic area”, to which all pain symptoms (PFDI 1, 2, 3, 6, 7 and 46) belonged, IPC was then defined as pain events occurring in a patient when all symptoms demonstrated R1 severity, while RPC was defined as pain events occurring in a patient when at least one of the six symptoms demonstrated R2 severity.
Group comparisons in terms of the frequency distribution of the symptom outcomes were performed on the basis of X2-tests or Fisher’s exact tests. Associations between symptoms with categorical data structure were also statistically evaluated with the X2-test for independence.
Global and simple effects of POP repair on the incidence of R2 and other specific outcomes/events of the considered pain symptoms were tested for significance with Cochran’s Q tests followed by McNemar localization tests.
Moreover, the effects of POP repair on R2 and regional dependent pain events were investigated under the additional consideration of location and degree of the anatomical POP defect.
| Results | ▴Top |
Baseline distribution of pain severity
Type-dependent pain
In view of the first hypothesis of the study, statistical analysis was focused on the baseline pain severity distribution within the total sample population and within some interesting subsamples as well.
Table 1 demonstrates the absolute and relative preoperative frequencies of the four pain intensities: “no or not at all” (symptom-free), “somewhat”, “moderate”, “quite a bit” and the combination of “moderate” or “quite a bit” (R2) for the six PFDI types 1, 2, 3, 6, 7 and 46 in the following groups: 1) total population (tP, n = 277); 2) anterior/apical POP (aaP, n = 142) and posterior/apical POP (paP, n =135); 3) stage 2 POP (S2P, n = 122) and stage 3 or 4 POP (S34P, n = 150).
 Click to view | Table 1. Baseline Prevalence of the Four Symptom Outcomes and the Combination R2 Regarding the PFDI Symptoms in Patient Groups |
In tP group (Table 1), the “symptom-free” outcome of the six PFDI types had a prevalence rate between 38.6% and 60.3%, a “somewhat” outcome between 15.20% and 22.1%, a “moderate” outcome between 13.4% and 20.9% and a “quite a bit” outcome between 13.04% and 22.7%.
All six pain sensations were found in patients with aaP, paP, S2P and S34P. Regarding R2, PFDI 6 was the most frequent pain type (40.8%), followed by PFDI 7 (37.9%), PFDI 1 (32.9%), PFDI 3 (27.1%), PFDI 46 (24.6%) and PFDI 2 (22.8%).
Patients with paP vs. aaP suffered significantly more from R2 complaints regarding the pain types PFDI 1 (37.0% vs. 28.9%), 2 (28.1% vs. 17.7%), 3 (34.1% vs. 20.5%) and 7 (43.0% vs. 33.1%) (Fisher’s exact tests, P < 0.05). Between 35.9% and 67.6% of women with aaP and 40.0-54.8% of women with paP were pain-free preoperatively.
Concerning the various pain types identified by PFDI 1, 2, 3, 6, 7 and 46, S2P vs. S34P patients reported R2 pain frequencies in the range of 23.8-39.4% vs. 21.4-42%. Considering all pain types, 18.4% of S2P patients exhibited “somewhat”, 19.1% “moderately” and 16.9% “quite a bit” complaints. In S34P patients, the average frequency distribution was “somewhat”, “moderately” and “quite a bit” in 18.7%, 6% and 14.3%, respectively. Pain types and intensity were not significantly different.
In summary we can say that about 40% of women with POP stage greater than 1 show in the baseline relevant pain severity (R2) by at least one pain type. When comparing the R2 prevalence of the various pain symptom types between paP and aaP as well as between S2P and S34P we found by four of the six pain symptoms significant differences between paP and aaP.
Location-related pain
In terms of location-related pain, the focus of the statistical analysis was on the two pain events IPC and RPC.
Concerning tP, a breakdown of pain complaint into anterior, visceral or posterior area resulted in RPC frequencies of 37.9%, 46.6% and 44% (Table 2). Evaluating the subgroups, the distribution of pain complaint to anterior, visceral or posterior in aaP was 34.5%, 49.0% and 38.7%; in paP, 41.1%, 50.4% and 49.6%; in S2P, 39.3%, 43.4% and 45.1%; and in S34P, 36.7%, 49.3% and 43.3%.
 Click to view | Table 2. Absolute and Relative Frequencies of the IPCs and RPCs in the Anterior, Visceral, Posterior and the Whole Pain Complaint Area (All Areas) for Different Patient Samples Preoperatively |
The anterior vs. visceral/posterior complaints showed the fewest RPC frequencies in tP and subgroups (Table 2). However, statistically significant differences were found entirely between the anterior and visceral complaints in the tP and S34P.
Furthermore, the data showed that aaP vs. paP patients suffered less frequently from RPC in all complaints (anterior + visceral + posterior) (61.2% vs. 74.1%) as well as in the anterior (34.5% vs. 41.5%), visceral (43.0% vs. 50.4%) and posterior (38.7% vs. 49.6%) complaints (Table 2). However, the differences were only statistically significant for all complaints (X2-tests, P < 0.05).
Interestingly, POP stage did not significantly influence the frequency of relevant pain in any of the considered pain complaints (Table 2).
Surgery and cure-duration effects on pain
POP-repair effects on type-dependent pain in the total sample population
Focusing first on the analysis of R0 (“symptom-free”) and R2 outcomes (“moderately” or “quite a bit”), all six pain types (Table 3) in the tP group were significantly reduced 6, 12 and 24 months after surgery. Twenty-four months postoperatively, the R0 state was most noted by PFDI 2 in 92.4%, followed by PFDI 3 in 91.9%, PFDI 6 in 91.4%, PFDI 1 in 88.1%, PFDI 46 in 84.3% and PFDI 7 in 75.1% (Table 3). The R0 outcome was noted in all postoperative phases, while the R2 outcome was reported significantly less than noted at baseline, indicating improvement in pain symptoms (Table 3).
 Click to view | Table 3. Absolute and Relative Frequencies of the Four Symptom Outcomes and the Combined Outcome R2 Before and 6, 12 and 24 Months After POP Reconstruction in the Total Population |
For each single outcome, the ranges of the prevalence rates over the six PFDI pain types showed considerable changes from baseline to 24 months after surgery. Thus, for the “symptom-free” outcome, the preoperative and 24-month postoperative prevalence ranges were 38.6-60.3% and 75.1-92.4% respectively; for the “somewhat” outcome, they were 15.2-22.1% and 3.8-6.5%; for the “moderately” outcome, 13.4-20.9% and 1.6-9.2%; and for the “quite a bit” outcome, 13.4-22.7% and 1.1-8.6%. Among the six PFDI pain types, PFDI 6 showed the highest prevalence rates for the “symptom-free” and “quite a bit” outcomes 24 months after surgery (about 80% increase and 94% decrease, respectively).
POP-repair effects on location-dependent pain in the total sample population
In the tP group, the RPC frequency regarding the complete pelvic area was reduced from 67.5% preoperatively to 24.9% 24 months postoperatively, which was equivalent to a success rate of 63.1% (Table 4). The success rate for anterior was 84.4%, for visceral 87.3% and for posterior area complaints 45.2%. The postoperative prevalence rates in all three areas were significantly lower than preoperatively. Furthermore, RPC occurred significantly less often in the anterior and visceral complaints compared to the posterior 6, 12 and 24 months after surgery. And the cure rates for pain with anterior vs. posterior and visceral vs. posterior complaints were also significantly better (Table 4).
 Click to view | Table 4. Pre- and Postoperative Observed Cases and Occurrences of RPCs in the Anterior, Visceral, Posterior and All Complaint Areas in the Total Population (n = 277) |
The findings of the above two sections confirm the first three hypotheses formulated in the introduction.
POP-repair effects on location-dependent pain in different subsamples
Repair effects in the aaP and paP groups
The pre- and 24-month postoperative prevalence rates of RPC in the paP subgroup were as follows: all pain complaints 74.1% vs. 31.8% (cure rate (cr) = 57.0%), anterior 41.5% vs. 9.1% (cr = 78.0%), visceral 50.4% vs. 9.1% (cr = 81.9%) and posterior 49.6% vs. 26.4% (cr = 50.4%) (Table 5).
 Click to view | Table 5. Absolute and Relative Frequencies (Prevalence Rates) of RPCs for Patients With Posterior/Apical POP (Group paP; n = 135) and Those With Anterior/Apical POP (Group aaP; n = 142) |
In the aaP subgroup, the pre- vs. 24-month postoperative prevalence rates of RPC were as follows: all pain complaints 61.3% vs. 14.7% (cr = 76.0%), anterior 34.5% vs. 1.3% (cr = 96.2%), visceral 43.0% vs. 1.3% (cr = 96.9%) and posterior 44% vs. 13.3% (cr = 65.6%). The paP patients as well as the aaP patients suffered significantly less often from RPC in each postoperative phase than at baseline, irrespective of the region where the RPC occurred. Comparing the aforementioned 24-month cure rates of aaP patients with those of paP patients in the three pelvic areas, better cure rates were found for aaP patients. All pain complaints noted 76.0% vs. 57.0% (19.0% better), anterior 96.2% vs. 78.0% (18.2% better), visceral 96.9% vs. 81.9% (15.0% better) and posterior 65.6% vs. 50.4% (15.2% better), respectively. The best cure effect was achieved in aaP patients with RPC visceral (96.9%) or anterior pains (96.2%), while the least cure effect was seen in paP patients with posterior pain (50.4%).
The inferential comparisons of aaP vs. paP patients in terms of the different pelvic areas supplied the following results. Preoperatively, paP vs. aaP patients suffered significantly more from RPC for anterior, visceral, posterior and all complaints. Twelve and 24 months after surgery, paP vs aaP patients had significantly lower RPC prevalence rates for any complaint (X2-tests, P < 0.05).
Repair effects in the S2P and S34P groups
The pre- and 24-month postoperative RPC prevalence and cure rates were as follows (Table 6): S2P: all complaints 45.1% vs. 35.2% (cr = 21.9%), anterior 39.3% vs. 9.9% (cr = 74.8%), visceral 43.4% vs. 8.8% (cr = 79.7%) and posterior 43.4% vs. 29.7% (cr = 31.5%) (Table 6); S34P: all complaints 43.3% vs. 15.7% (cr = 63.7%), anterior 36.7% vs. 2.2% (cr = 94.0%), visceral 49.3% vs. 3.4% (cr = 93.1%) and posterior 49.3% vs. 13.5% (cr = 72.6%).
 Click to view | Table 6. Absolute and Relative RPC Frequencies (Prevalence Rates) of Patients With Stage 2 POP-Q (Group S2P; n = 122) and Stage 3 or 4 POP-Q Stage (Group S34P; n = 150) |
The comparison of the S34P vs. S2P groups in terms of the RPC cure rates after 24 months revealed the following differences (improvements): all complaints 63.7% vs. 21.9% (41.8% improvement), anterior 94.0% vs. 74.8% (19.2% improvement), visceral 93.1% vs. 79.7% (13.4% improvement) and posterior 72.6% vs. 31.5% (41.1% improvement). The best cure effect was achieved in the S34P patients with RPC anterior pain (94.0%) or visceral pain (93.1%), and the least cure effect was found in S2P patients with posterior pain (31.5%). Similar to the aaP and paP patients, the S2P as well as the S34P patients suffered significantly less often from RPC in each postoperative phase than at baseline, irrespective of pain region.
Compared with X2-tests of the RPC prevalence rates of S2P vs. S34P patients in each region, the results demonstrate that S34P patients vs. S2P patients showed a significantly stronger reduction in RPC for anterior, posterior and all complaints at 12 and 24 months after surgery, but interestingly not at 6 months postoperatively.
Surgery effects concerning pain severity changes
To obtain a better impression of the severity changes for each type of pain, a particular look at the “quite a bit” outcome shows the following results (Table 3).
Calculating the cure rates of the “quite a bit” severity before and 24 months after surgery for the various PFDI types in tP (calculations were based on the prevalence rates at these phases), we obtained 93.1% for PFDI 1, 88.3% for PFDI 2, 91.5% for PFDI 3, 94.5% for PFDI 6, 62.1% for PFDI 7 and 57.0% for PFDI 46. The prevalence rates of “somewhat” and “moderately” intensity outcomes were also reduced. The prevalence rates of the “symptom-free” outcome (Table 3) increased significantly from baseline to 24 months after surgery. These facts provide evidence for the curative power of POP reconstruction with respect to pain symptoms.
Pain severity transitions between pre- and postoperative phases
Figure 1 illustrates the transition structure for IPC and RPC between these states in the anterior, visceral and posterior area when passing over from baseline to one of the three postoperative phases. According to its definition, RPC means that at least one of the pair symptoms belonging to the aforementioned pain complaint areas show the outcome R2.
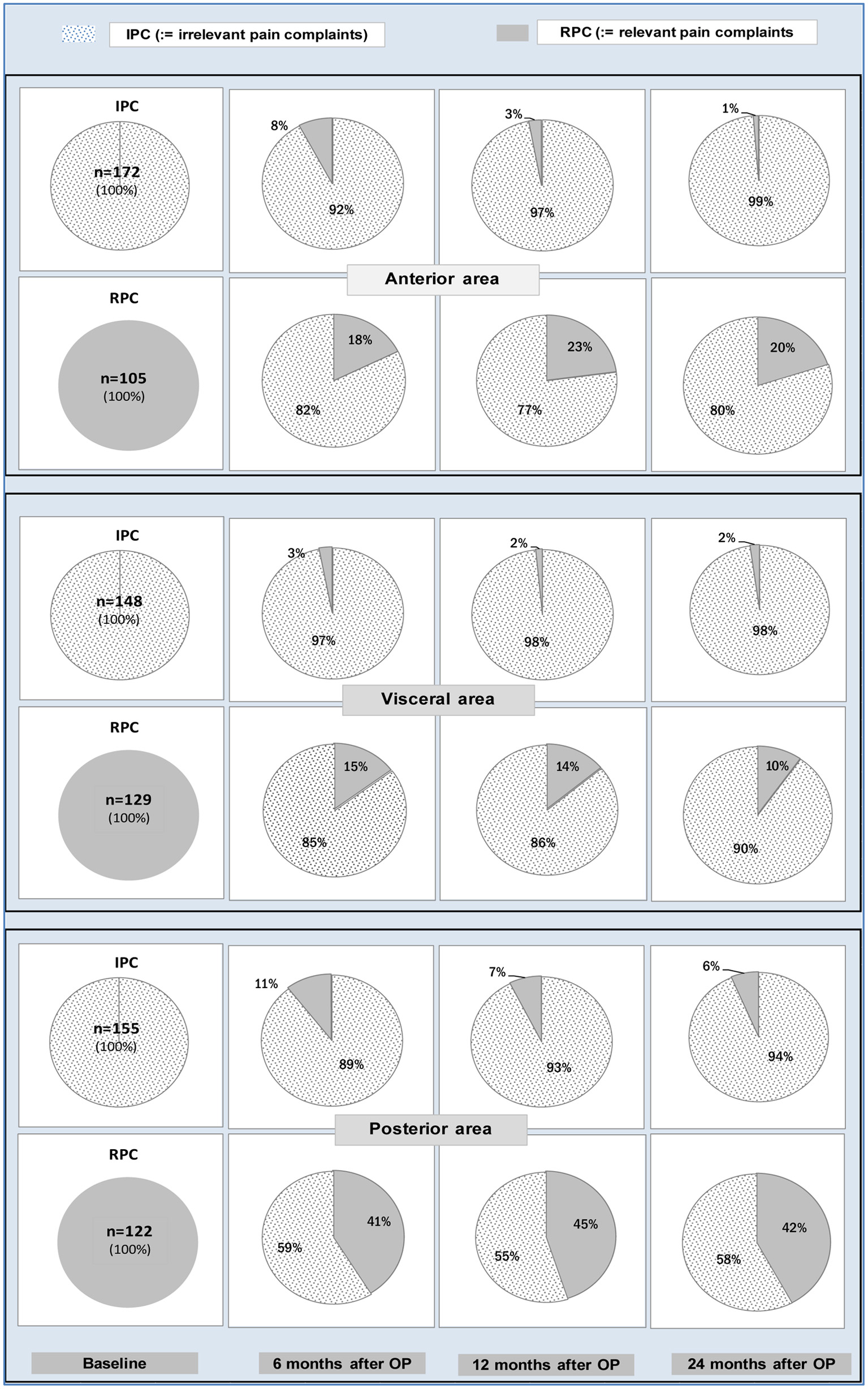 Click for large image | Figure 1. Proportions of patients who showed preoperatively the IPC state (bottom row of pies) or the RPC state (lower row of pies) for each pain complaint area and who stayed on the same or passed over to the other state 6, 12 or 24 months after surgery. Obviously, patients suffering from anterior or visceral pain at baseline showed better results 6, 12 or 24 months after surgery than patients suffering from posterior pain. IPC: irrelevant pain complaint; RPC: relevant pain complaint. |
Surgery-induced neo-pain
Preoperatively, 55 patients had no RPCs, whereas 6 months after surgery, nine patients (16.3%) suffered from RPCs, six (10.9%) patients 12 months after surgery and only four patients (7.2%) 24 months after surgery. This demonstrates that operatively induced pain decreases to a low level over time.
Associations and comorbidities
By testing the association between POP stage and pain intensity with X2-independence tests, no significant correlation among any of the considered pain types was found. Regarding the R2 outcome group and the presence of coexisting pelvic floor symptoms preoperatively, “daytime urinary frequency” was seen in 48.0% of the patients, “urgency” in 47.3%, “urgency incontinence” in 38.2%, “nocturia” in 48.7%, bladder emptying problems in 31.4%, stress urinary incontinence in 19.9%, fecal incontinence in 17.7% and stool outlet obstruction in 6.1%. The cure rates for these coexisting symptoms noted at 24 months after surgery ranged between 59% and 84%, as previously reported [23].
| Discussion | ▴Top |
The aim of this article was not to list all the numerous causes for CPP but to note the relationship between POP and pelvic pain and the potential resolution of that pain following reconstructive POP surgery. In the Propel study, the primary endpoint was confirmed noting that reconstructive POP surgery with the use of mesh restored normal anatomy (POP-Q ≤ 1) in a statistically significant percentage of patients [30, 31]. The secondary study endpoints were likewise illustrated, as POP-induced bladder and anorectal symptoms were often improved or cured by surgical POP repair [9, 32]. The tertiary, so far not evaluated endpoint presented in this paper was to determine the relationship between POP and pain before and after reconstructive surgery, with respect to type, location, severity and frequency.
The preoperative data evaluation showed that two-thirds of all POP patients (67.5%) had RPCs in the complete pelvic area, while only one-third did not. In the tP as well as in the subgroups, the visceral complaints were the main pain, followed by the posterior and anterior.
An explanation for this finding seems to be that the upright position of human beings forces the sacrum to curve in an age-dependent manner [33]. The more the sacrum curves, the more the pelvic floor comes in a horizontal position. Since the inclination angle of the pelvic floor determines the degree of the forces acting on it, the center of the now horizontal shape is increasingly under pressure and gives way to prolapse. And if pressure becomes stronger, then overstretched connective tissue, ligaments, nerves and muscles react with pain [33].
There are two pathways of pain transmission for CPP described by Martius [34]: a visceral and a mechanical pathway. The visceral pathway is transmitted from Frankenhauser’s plexus, located in the middle of the pelvis in the parametrium, approximately 2 cm lateral to the cervix. The paired ganglia undergo permanent stimulation, if the uterus or vagina descend, and can cause serious pain, similar to pain during childbirth [2]. The pain radiates mainly to the anterior and lateral abdominal wall, the inguinal region and the thighs. This is the pathway for visceral and anterior pain. The mechanical pathway is transmitted by deficient, overstretched suspensory ligaments or support from the pelvic floor leading to increased tension against the sacral plexus, thereby causing pain.
In this context, in 2015, Goeschen [8] pointed out that mechanical support of the uterus and vagina by restoration of the supportive and suspensory structures should be able to stop the permanent stimulation of the paired ganglia. These patients should be free of prolapse-related pain unless the supporting system gives way again.
The results of this study are in line with the prior considerations of Goeschen [8]. The postoperative data showed the following. In the total population, all six pain types were significantly reduced 6, 12 and 24 months after reconstructive POP surgery. The visceral symptom area had the highest pain reduction rate (87.3%), followed by the anterior (84.4%) and the posterior area (45.2%) after surgical POP repair. The most bothersome pain complaint category, “quite a bit”, noted the highest improvement rate of 80.5%.
The cure rates for visceral/anterior vs. posterior pain complaints were significantly better with very small odds ratios of RPCs, indicating a very low risk for recurrence.
Cystocele/rectocele
Patients with cystocele (aaP) vs. recto/enterocele (paP) had significantly less severe pain in all three areas preoperatively. The best cure effects after surgical POP repair were achieved in aaP patients with middle or anterior pain (> 96%), while paP patients with posterior pain noted a much lower cure effect (50%). This means that paP patients had significantly more pain than aaP patients pre- and postoperatively. Surgery in patients with pain in the posterior region was associated with limited results in both groups, especially in paP patients. How can this be explained?
Visceral and anterior pains are mainly transmitted via descended Frankenhauser ganglia. If these paired ganglia are surgically returned to a normal position, patients can appreciate improvement, if not persistence of their pain.
The second mechanical pathway described by Martius [34] is the main transmission pathway for posterior pain. This type of pain radiates primarily to the lumbosacral region and is characterized by low dragging abdominal pain or deep sacral backache.
Three mechanical changes can cause these pains: 1) Deficient suspensory ligaments. Deficient suspension of the visceral organs in the pelvic cavity due to loose ligaments can lead to serious tension on the sacral plexus, resulting in severe posterior pain in this area [8]; 2) Deficient support from the pelvic floor. The contents of the small pelvis are also supported from the base. A decline in the support of the pelvic floor followed by the unavoidable descent of pelvic organs causes tension on the suspending ligaments. This can generate pain in the lumbosacral area, primarily initiated by the deficient pelvic floor [8]; 3) Pain induced by overstretching the uterosacral ligaments (USLs). The nerve fibers in the uterosacral ligaments are parasympathetic visceral fibers. Stretching of lax ligaments via gravity may stimulate the nerve fibers within these tissues and cause pain [8].
All three possibilities lead to a stretching of either the nerve endings or muscle fibers within the USL, generating traction against the sacral plexus [8].
Because all three possibilities can be responsible for posterior pain, all damaged structures have to be repaired. Otherwise, pain remains or returns.
The posterior reconstruction performed in this study was obviously not sufficient enough to restore the suspending and supporting system sufficiently. Therefore, the cure rate of posterior pain in tP patients was only 45%. In contrast, the elevation of prolapsed Frankenhauser ganglia led to a pain cure rate of more than 84% in tP patients with visceral or anterior complaints.
POP stage
Preoperatively, no correlation was found between POP stage and pain intensity. Therefore, the expectation that patients with higher POP stages have more severe pain could not be verified. This finding could be attributed to a patient’s sense of accommodation to chronic pain over time, resulting in a decreased perception of pain intensity in women with more severe POP.
However, with respect to pain cure rates, the best cure rates (> 93%) were noted in patients with pronounced POP (S34P) suffering from visceral or anterior pain. By comparison, only limited cure rates (31.5%) were seen in S2P patients with posterior pain. These findings suggest that S34P patients stand to benefit significantly more with respect to improvement or resolution of pain symptoms from reconstructive surgery than those with smaller POP.
De novo pain
In the literature, the reported incidence of de novo pain after POP repair is variable [1-3]. In a study following the use of Elevate (American Medical Systems) or Prolift (Gynecare/Ethicon) kit-based POP repair systems, pelvic pain was noted in 11% of patients [1]. Kowalik et al reported only 5% de novo pain after POP mesh surgery [2]. The true incidence is likely underreported since 38.6% of complaints to the FDA pertained to vaginal pain and/or dyspareunia; however, a recent Cochrane review in 2013 noted only 0.5% of patients undergo mesh removal for pain [3].
In our study, surgically induced de novo pain was low and decreased over time from 16% at 6 months, 11% at 12 months and 7% at 24 months postoperatively, and the prevalence rate of “quite a bit” complaints was only 0-6%. This shows that patients suffering from de novo pain after POP reconstruction, even when mesh is used, should proceed with conservative, non-operative management rather than rush to a surgical intervention, as neo-pain often improves and can even resolve over time.
Limitations of the study
The data are derived from a study group of women who decided to undergo prolapse repair for symptomatic POP.
Comorbidities
In 1993, Petros and Ulmsten described CPPS as being caused by lax uterosacral ligaments as part of the “posterior fornix syndrome” [12], along with other pelvic symptoms such as nocturia, urgency and abnormal emptying. They reported a significant cure rate of CPPS and other posterior fornix symptoms following repair of the uterosacral ligaments and vaginal apical reconstruction [12].
In 2003, Van Os-Bossagh et al [35] reported that 43% of 60 women with CPP had serious urinary incontinence, 10% had an urge component, 25% had pure stress urinary incontinence and 8% had unclassified incontinence.
In a recently published paper, Goeschen [36] reported a 30-40% coexistence of bladder and bowel dysfunctions in patients with CPP. All symptoms improved significantly following surgical repair of the pelvic floor.
In the present study, nearly identical data regarding comorbidities were observed. This underlines the Iceberg concept of Pescatori [37], who pointed out that patients usually come for treatment with one main symptom, while other symptoms, though present, may be latent.
Pain severity changes
The illustration of pain severity changes for the six different pain types (from baseline to 24 months after surgery) shows that the most bothersome severity level “quite a bit” (94%) had the highest cure rates 24 months after repair regarding PFDI types 1, 2, 3 and 6. For PFDI types 7 and 46, the pain cure rates were less, but still 49% and 62%, respectively. Once again, pain perceived to be “visceral” or “anterior”, benefits significantly more from POP reconstructive surgery than pain perceived to be “posterior”.
Hypotheses in the introduction chapter
Reflecting upon the hypotheses in the introduction chapter regarding type-dependent pain in the total sample and the subgroups it can be summarized that POP reconstruction causes a relevant improvement in pain severity for a long time irrespective of pain type with the best results for visceral complaints.
Conclusion
The present findings provide further evidence that POP causes relevant pelvic pain in two-thirds of patients. Appropriate anterior/apical or posterior/apical POP reconstruction enables resolution of the six pain types in 75-92% of patients, with a long-lasting durability of response. The visceral complaints had the highest pain reduction rate (87%) after surgical POP repair, followed by the anterior (84%) and the posterior (45%). The surgical principle of re-establishing vaginal apical support may provide a primary surgical option for the cure of pelvic pain experienced concomitantly with prolapse. The use of alloplastic material and the restoration of the correct vaginal axis towards S2-S4 seem to be important for a durable cure. Preoperative simulated operations and the use of a diagnostic algorithm, such as that previously proposed by Petros [38-40], may be helpful. All available techniques for POP repair should be evaluated in the context of pain relief and activation so that the effects of different techniques on pain can be adequately compared. While women with relevant preoperative pain have a good chance of pain relief after mesh-supported vaginal POP-repair, women without preoperative relevant pain can develop relevant pain, which should be considered in preoperative indication for type of surgery with or without alloplastic material.
Acknowledgments
We would like to thank the following surgeons for their contributions to data collection: James C. Lukban, Edward Stanford, James Flaherty, Robert Moore, Jan-Paul Roovers, Seth Herbst, M. Virelles, Manish Patel, Roger Beyer, Ty Erickson, Nguyen Kim Nguyen, Samuel Zylstra, Douglas VanDrie, Thomas Giudice, Veronica Mallett, Daniel Biller, Kurt McCammon, Labib Riachi, Christophe Cortieu, Christopher Mayne, Pr. Dirk De Ridder, Suzette E. Sutherland, Bernhard Liedl, Eduardo Bataller, Carlos Medina, Philip Hoekstra and Rainer Lange.
Financial Disclosure
Data were collected from an industry sponsored (AMS) 2008-12 multicenter “Propel” study.
Conflict of Interest
B. Liedl: consultant - American Medical Systems, Boston Scientific; research - American Medical Systems. K. Goeschen: none. N. Grigoryan: none. S. Sutherland: Consultant - American Medical Systems, Boston Scientific, Axonics; research - American Medical Systems, FemPulse, Pelvital. A. Yassouridis: none. M. Witczak: none. JP Roovers: consultant - American Medical Systems, Coloplast, Tepha, Promedon; research - American Medical Systems, Coloplast, Tepha.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author Contributions
BL: project development, data collection, management data analysis and manuscript writing. KG: manuscript writing and management data analysis. NG: manuscript writing and data analysis. SES: data collection, manuscript writing and editing. AY: statistics and data analysis. MW: manuscript writing and editing. JPR: data collection and editing.
Data Availability
Dr. Yassouridis and all other authors analyzing the data got the raw data of the whole study.
| References | ▴Top |
- Rogowski A, Bienkowski P, Tarwacki D, Szafarowska M, Samochowiec J, Sienkiewicz-Jarosz H, Jerzak M, et al. Retrospective comparison between the Prolift and Elevate anterior vaginal mesh procedures: 18-month clinical outcome. Int Urogynecol J. 2015;26(12):1815-1820.
doi pubmed - Kowalik CR, Lakeman MME, Oryszczyn JE, Roovers J. Reviewing Patients Following Mesh Repair; The Benefits. Gynecol Obstet Invest. 2017;82(6):575-581.
doi pubmed - Maher C, Feiner B, Baessler K, Schmid C. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;4:CD004014.
doi - FDA. Urogynecologic surgical mesh implants. https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants.
- Klinge U, Park JK, Klosterhalfen B. 'The ideal mesh?'. Pathobiology. 2013;80(4):169-175.
doi pubmed - Klosterhalfen B, Klinge U. Retrieval study at 623 human mesh explants made of polypropylene—impact of mesh class and indication for mesh removal on tissue reaction. J Biomed Mater Res B Appl Biomater. 2013;101(8):1393-1399.
doi pubmed - Toozs-Hobson P, Cardozo L, Hillard T. Managing pain after synthetic mesh implants in pelvic surgery. Eur J Obstet Gynecol Reprod Biol. 2019;234:49-52.
doi pubmed - Goeschen K. Role of uterosacral ligaments in the causation and cure of chronic pelvic pain syndrome. Pelviperineology. 2015;34:2-20.
- Liedl B, Goeschen K, Durner L. Current treatment of pelvic organ prolapse correlated with chronic pelvic pain, bladder and bowel dysfunction. Curr Opin Urol. 2017;27(3):274-281.
doi pubmed - Martius H. Uber einen haufigen gynakologischen Symptomkomplex. Archives of Gynecology and Obstetrics. 1938;166:332-335.
doi - Martius H. Die Kreuzschmerzen der Frau, ihre Deutung und Behandlung. Thieme, Leipzig. 1939.
- Petros P, Ulmsten U. The posterior fornix syndrome: a multiple symptom complex of pelvic pain and abnormal urinary symptoms deriving from laxity in the posterior fornix. Scandinavian Journal of Urology and Nephrology. 1993;27(Suppl. 153 - PART IV):89-93.
- Clemens JQ, Kutch JJ, Mayer EA, Naliboff BD, Rodriguez LV, Klumpp DJ, Schaeffer AJ, et al. The Multidisciplinary Approach to The Study of Chronic Pelvic Pain (MAPP) Research Network*: Design and implementation of the Symptom Patterns Study (SPS). Neurourol Urodyn. 2020;39(6):1803-1814.
doi pubmed - AWMF-Leitlinie, Chronischer Unterbauchschmerz der Frau. Register-Nr. 016/001. 2008. https://www.awmf.org/uploads/tx_szleitlinien/016-001l_S2k_Chronischer_ Unterbauch-schmerz_Frau_2016-06.pdf.
- Kavvadias T, Baessler K, Schuessler B. Pelvic pain in urogynecology. Part II: treatment options in patients with lower urinary tract symptoms. Int Urogynecol J. 2012;23(5):553-561.
doi pubmed - Dwyer PL. Chronic pelvic pain in urogynecological practice: a personal view. Int Urogynecol J. 2011;22(4):383-384.
doi pubmed - Malde S, Palmisani S, Al-Kaisy A, Sahai A. Guideline of guidelines: bladder pain syndrome. BJU Int. 2018;122(5):729-743.
doi pubmed - Heit M, Culligan P, Rosenquist C, Shott S. Is pelvic organ prolapse a cause of pelvic or low back pain? Obstet Gynecol. 2002;99(1):23-28.
doi pubmed - Fall M, Baranowski AP, Elneil S, Engeler D, Hughes J, Messelink EJ, Oberpenning F, et al. EAU guidelines on chronic pelvic pain. Eur Urol. 2010;57(1):35-48.
doi pubmed - Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87(3):321-327.
doi - Jamieson DJ, Steege JF. The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstet Gynecol. 1996;87(1):55-58.
doi - Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287-333.
doi pubmed - Evans SF. Chronic pelvic pain in Australia and New Zealand. Aust N Z J Obstet Gynaecol. 2012;52(6):499-501.
doi pubmed - Engeler D, Baranowski AP, Borovicka J, Cottrell AM, Dinis-Oliveira P, Elneil S, Hughes J, et al. EAU guidelines on chronic pelvic pain. EAU Guidelines Office, Arnhem, The Netherlands. 2018. http://uroweb.org/guidelines/compilations-of-all-guidelines/.
- Williams RE, Hartmann KE, Steege JF. Documenting the current definitions of chronic pelvic pain: implications for research. Obstet Gynecol. 2004;103(4):686-691.
doi pubmed - The American College of Obstetrics and Gynecologists. FAQ099, August 2011 https://m.acog.org/Patients/FAQs/Chronic-Pelvic-Pain.
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187(1):116-126.
doi pubmed - Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167-178.
doi pubmed - Clinicaltrials. https://clinicaltrials.gov/ct2/show/study/NCT00638235?cond=NCT00638235&rank=1.
- Stanford EJ, Moore RD, Roovers JP, Courtieu C, Lukban JC, Bataller E, Liedl B, et al. Elevate anterior/apical: 12-month data showing safety and efficacy in surgical treatment of pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2013;19(2):79-83.
doi pubmed - Lukban JC, Roovers JP, Vandrie DM, Erickson T, Zylstra S, Patel MP, Moore RD. Single-incision apical and posterior mesh repair: 1-year prospective outcomes. Int Urogynecol J. 2012;23(10):1413-1419.
doi pubmed - Liedl B, Goeschen K, Sutherland SE, Roovers JP, Yassouridis A. Can surgical reconstruction of vaginal and ligamentous laxity cure overactive bladder symptoms in women with pelvic organ prolapse? BJU Int. 2019;123(3):493-510.
doi pubmed - Goeschen K, Liedl B. Chronic pelvic pain and pelvic organ prolapse: A consequence of upright position? Pelviperineology. 2020;39(2):55-59.
doi - Martius H. Geschlechtseigentumliche gynakologische Schmerzen. In: Thieme, editor. Lehrbuch der Gynakologie. Stuttgart. 1946:85-96.
- van Os-Bossagh P, Pols T, Hop WC, Bohnen AM, Vierhout ME, Drogendijk AC. Voiding symptoms in chronic pelvic pain (CPP). Eur J Obstet Gynecol Reprod Biol. 2003;107(2):185-190.
doi - Goeschen K, Gold DM. Surgical cure of chronic pelvic pain, associated bladder & bowel symptoms by posterior sling in 198 patients validates the Pescatori Iceberg principle of pelvic symptom co-occurrence. Pelviperineology. 2017;36:1-4.
- Pescatori M, Spyrou M, Pulvirenti d'Urso A. A prospective evaluation of occult disorders in obstructed defecation using the 'iceberg diagram'. Colorectal Dis. 2007;9(5):452-456.
doi pubmed - Petros PE, Ulmsten UI. An integral theory and its method for the diagnosis and management of female urinary incontinence. Scand J Urol Nephrol Suppl. 1993;153:1-93.
- Petros P. The integral theory system. A simplified clinical approach with illustrated case histories. Pelviperineology. 2010;29:37-35.
- Liedl B, Inoue H, Sekiguchi Y, et al. Update of the integral theory and system for management of pelvic-floor dysfunction in females. Eur Urol Suppl. 2018;17:100-108.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
